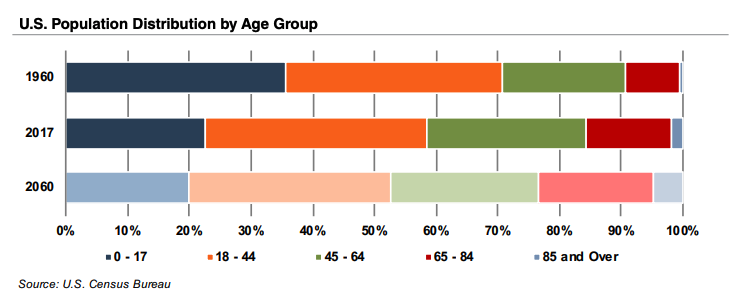2022 Update: Medicare and Medical Devices
Medical device manufacturers (MDMs) spend a lot of time and resources on their FDA approval strategy, and rightly so. It is the most critical milestone in a go-to-market strategy, and approval for medicare reimbursement is not far behind.
Grandview Research estimated the 2020 global medical devices reimbursement market at USD 374.4 billion in 2020. They predict it will expand at a compound annual growth rate (CAGR) of 10.8% from 2021 to 2028. Hospitals led the medical device reimbursement market in 2020, capturing 53% of the revenue share.
After the brief downturn during COVID-19, the medical device industry is on a rebound that could last for many years, driven by strong trends that key off of projected growth in the aging population. The U.S. Census Bureau estimates that the elderly will almost double by 2060 to 95 million, representing 23% of the total population.
In the US, healthcare and aging are synonymous with Medicare. An aging population leads to more demand for medicare reimbursed devices. It stands to reason that approval from the Centers for Medicare and Medicaid Services (CMS) is almost as important as FDA approval. It is difficult to sell a device to Healthcare Delivery Organizations (HDOs) DOs or providers without CMS reimbursement approval.
Medicare and Medicaid are significant because they reimburse HDOs, health care providers, and patients. Devices that do not meet the criteria for reimbursement are at a considerable disadvantage in the marketplace.
Let’s look a how Medicare influences MDMs at all stages of device deployment and company growth.
The Case for a Reimbursement Strategy
A reimbursement strategy is an MDMs plan for obtaining CMS reimbursement approval. The main reason to consider a reimbursement strategy is that devices approved for the private payer or Medicaid/Medicare reimbursements are easier to sell once they are on the market. Future sales potential is one reason why a reimbursement strategy should be part of the overall development strategy from the beginning, certainly before the company finalizes the development process.
The reimbursement strategy may impact clinical trials that companies need to perform prior to entering the market. Navigating the path to reimbursement can be complex and specialized. Some MDMs employ a reimbursement consultant to help them create their strategy.
The challenge for MDMs is that even after they get FDA approval, it can take years to secure CMS approval for reimbursement. One industry expert in 2020 said that approval for new procedure reimbursement typically takes three years, a clinical study, plus additional funds ranging from a modest fee to the hundreds of thousands of dollars.
At first glance, it would seem like FDA approval should carry more weight with the CMS. In reality, the two agencies have very different motivations. The FDA is concerned with consumer safety. CMS (and private insurers) primarily look at a device’s “health economic value” – efficiency, effectiveness, and value. That is why their approval process looks very different.
Considerations for a Reimbursement Strategy
Keeping the CMS bias toward economics in mind, here are some early questions to ask to build your reimbursement strategy:
- Will the device be cost-effective for the payer, reduce costs, and improve health outcomes?
- Will it be affordable to a wide range of hospitals and providers?
- Is the device for inpatient or outpatient use?
- What FDA regulatory path did the company use?
- Is there evidence that physicians and health care providers will use the product?
Going forward, there are several factors to consider as well. Here are some strategy tips from reimbursement consultants:
Insurance companies and CMS may require different parameters than the FDA does. Define these early to prevent needing to repeat costly tests to satisfy reimbursement requirements.
Understand the reimbursement process from the HDO’s point of view. Understand the applicable reimbursement medical codes for your device. This page provides detailed information about how hospitals and providers submit reimbursement claims for medical device usage.
In terms of creating your strategy for CMS approval, it is definitely easier, faster, and less expensive to build on what is accepted than trying to re-invent the wheel. Research your competition or niche. Does your medical device have functional similarities to what is already on the market? Research medical billing codes and see what reimbursement groups exist that may be a match for your device.
Do payers restrict reimbursement for similar devices based on coverage, indications, or specific sub-segments of the population?
Understand what supporting evidence you will need to provide to payers – government or private- to gather that evidence as you develop and not have to backtrack.
Develop relationships with medical facilities that may use your device.
Set up payer advisory boards at the early stages of product development.
Embed economic endpoints to your clinical studies and publish clinical and economic data in peer-reviewed journals to build credibility.
MCIT: A Failed Attempt to Fastrack CMS Approval
Unfortunately for MDMs, the FDA and CMS do not collaborate, and the CMS process for approval can take years.
In January 2014, the CMS released a rule intended to speed up access to innovative medical devices, called “breakthrough” devices. “Breakthrough” medical technologies treat life-threatening or irreversible conditions with limited to no treatments.
The CMS intended the rule to help Medicare patients with life-threatening or debilitating conditions who had no or limited treatment options.
The rule, Medicare Program; Medicare Coverage of Innovative Technology (MCIT) and Definition of ‘Reasonable and Necessary” stated that FDA approval would automatically trigger four years of Medicare approval for designated breakthrough devices, starting on the date of FDA approval, or a date chosen by the MDM within two years of authorization.
The rule also detailed new standards for “reasonable and necessary.” The CMS initially set the rule to take effect on March 15 but then delayed it twice, to May 15 and finally to Dec. 15.
On June 23, 2021, legislators introduced a bipartisan bill requiring Medicare to enact MCIT and cover breakthrough devices after approval from FDA. The bill met with strong resistance from the US insurance industry, no doubt because in its original format, the bill extended the four-year coverage mandate to private insurers as well after the initial roll-out with Medicare.
The medical technology industry trade association Advanced Medical Technology Association (AdvaMed) submitted comments to the CMS on October 15, 2021, including:
“We are discouraged that CMS has proposed to repeal the MCIT final rule, which would expedite access to breakthrough diagnostic and therapeutic devices for Medicare beneficiaries suffering from debilitating conditions, such as heart disease, diabetes, kidney disease, acute infections, sepsis, and cancer, which are prevalent in the Medicare population and represent a significant burden of disease, as well as societal cost.”
On November 12, 2021, the CMS rescinded the MCIT final rule, with CMS Administrator Chiquita Brooks-LaSure citing concerns the final rule may not have been sufficient to protect Medicare patients:
Although we continue to be in favor of enhancing access to new technologies, we are mindful that they may have unknown or unexpected risks and must first ensure such technologies improve health outcomes for Medicare beneficiaries. The Medicare program needs to implement policies that balance access and appropriate safeguards.
Going forward, CMS will explore approval process improvements but with “appropriate health and safety protections in place for the Medicare population.” CMS officials stated they would be working with many stakeholders, including the FDA, the Agency for Healthcare Research and Quality, MDMs. They will also hold public meetings in 2022 to gather opinions and public comments.
Moving Ahead
From the MDMs point of view, the level of coordination between the FDA and CMS is basically back where it started. As the number of new devices and patient demand for better access increases, political stakeholders may be motivated to streamline the CMS regulatory pipeline.
In the meantime, best practices for MDMs also take us back to where we started with this post. Be sure to include a reimbursement strategy in your development plans from the very beginning. We know that most MDMs didn’t get into the business because they have a passion for tackling regulatory hurdles and political interests. Feel free to contact us for support and help with questions about your regulatory journey.