Design Considerations to Maximize Cloud Connectivity
Cloud connectivity is becoming more popular for medical devices.
There are multiple benefits to connecting a medical device that makes it worth the investment for many.
If you’re thinking about whether to connect a medical device to the cloud and utilize powerful data-gathering potential, there are a few things to consider with regard to design:
Definition of connected medical device
First of all, how do we define a connected medical device? A medical device itself is a wearable or implantable electromechanical gadget that can either sense physiological patient data, or apply a therapy (such as a neurotransmitter).
A medical device has data input and/or data output. It can also include “near-patient” apparatus, such as a CPAP machine.
Connected devices are those that use computer networks to transfer, manage, store, and analyze health data.
Medical device data
There are four main types of medical device data:
- User physiological data. This is “output data,” such as vital signs – blood pressure, body temperature, brain activity, etc.
- User therapeutic data. This is “input data” to the medical device and includes any information that sets the therapeutic parameters.
- User demographic data. This is “output data” that includes user information that is not directly measured by the medical device, such as height, age, sleep activity, and weight.
- Device status data. This is “output data” to measure the health of the device itself. For example, it might include battery health data. As we often say, there is no “check engine” light for medical devices that aren’t connected. Cloud connectivity can provide the missing piece that helps with device monitoring.
The goals of your medical device
Often in the early stages of development, medical device companies have a strong focus on the core functionality of the device, A lot of time is spent working through design controls, device verification, and clinical trial validation.
Naturally, the company aims to get through market clearance, to prove to regulators that they have designed a safe, effective medical device that does what it says on the label. Regulatory and quality standards are rigorous, so naturally, in the early stages of a device, there can be a preoccupation with those.
Getting a device to market should not be the ultimate goal, in our view. The real objective should be market acceptance, which is where the device proves to have high usability. Typically, the market demands connectivity and the added benefits that can bring. We’re finding that connectivity is no longer a “nice to have” but more of a “need to have” for market acceptance. This means it should be planned for in the early days of your design.
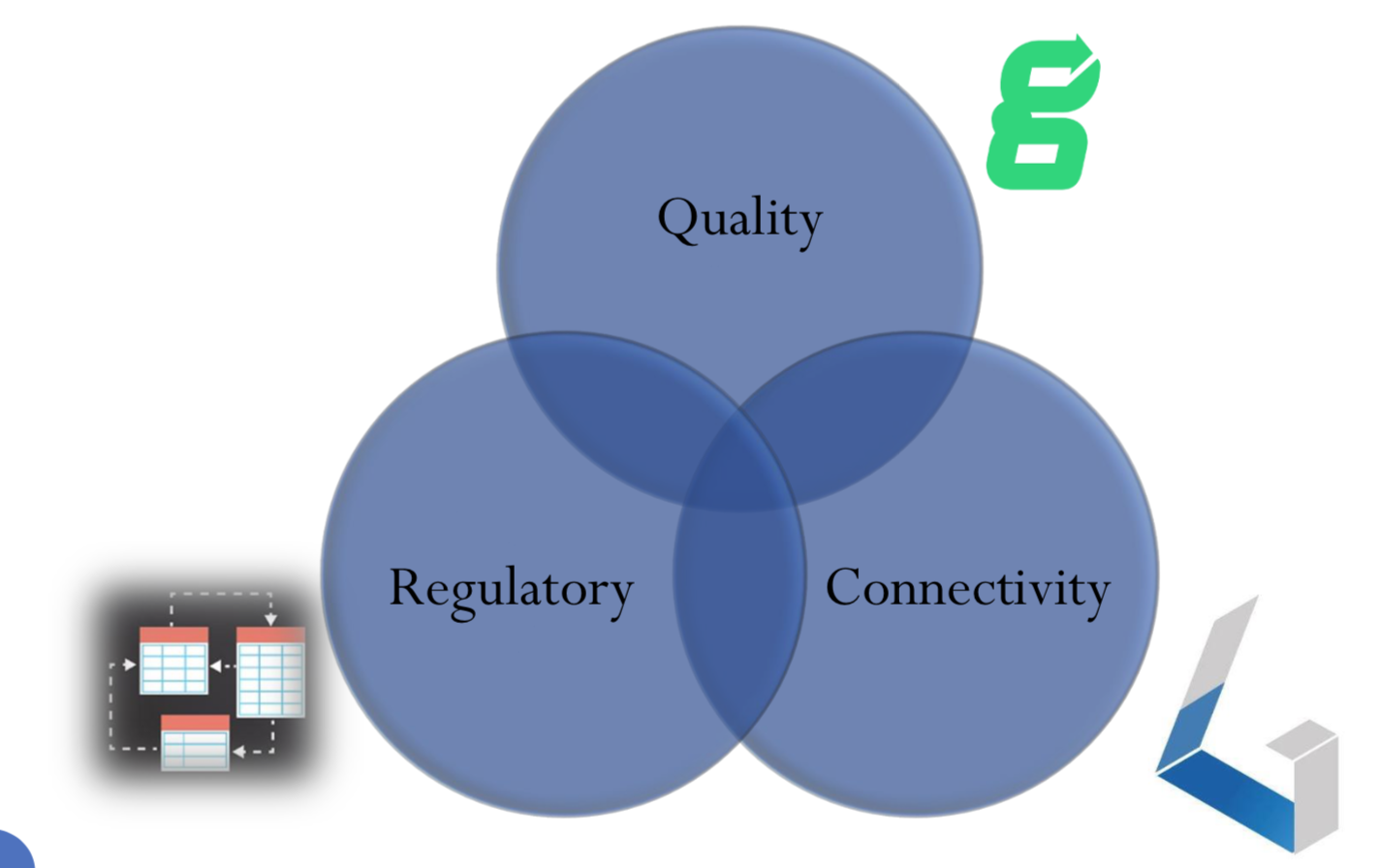
Key factors for market acceptance
There are three key factors for market acceptance. Each of them overlaps with and complement one another:
- Regulatory. As we touched on already, regulatory is a huge part of getting your medical device to market. If you don’t yet have a regulatory expert on your team, we would strongly suggest either hiring one in or finding a consultant who can help to guide you through the process.
- Quality. Every medical device should have a strong quality management system (QMS). This is a structured system encompassing your processes and procedures for all the key areas of your device development. For example, design controls, risk management, manufacturing, and supplier management. Our partners Greenlight Guru are experts in this area and have excellent software to help medical device companies to have a streamlined process in place.
- Connectivity. As mentioned, the ability for medical devices to store, share, or process data is in high demand. Having this functionality opens up new conveniences and possibilities for patient care.
Three key design questions for device companies
There are three key design questions that every medical device company should ask themselves with regard to connectivity:
#1. Should my device connect at all?
Risk is one consideration that medical device companies often raise with regard to connectivity. It’s true that connectivity is not without some risk. Primarily, those are related to privacy or cybersecurity violations.
However, it is possible to minimize that risk. If you follow best practices, including security monitoring, regular updates, and following HIPAA guidelines, then it is unlikely that you will be faced with either of those risks in reality.
From our perspective, there is more risk in NOT connecting a medical device. For example, if you put yourself in the shoes of a parent of an epileptic child, a connected device can alert them if their child is out with friends and has a seizure. They could take action immediately. On the other hand, if they didn’t have a connected device, there could be delays in the parent being notified.
Another example is with medical devices that can monitor medication dosage and the circumstances surrounding the correct dosage for the patient. Connectivity brings advantages, such as being able to analyze data and alert when underdosing might be occurring. Without this, the patient might wait months for their next physician appointment to find that they have been underdosing and have returned to a disease state.
Here at Galen Data, we’ve created a platform to reduce connectivity risk, by implementing industry-leading access controls and fundamental security design features.
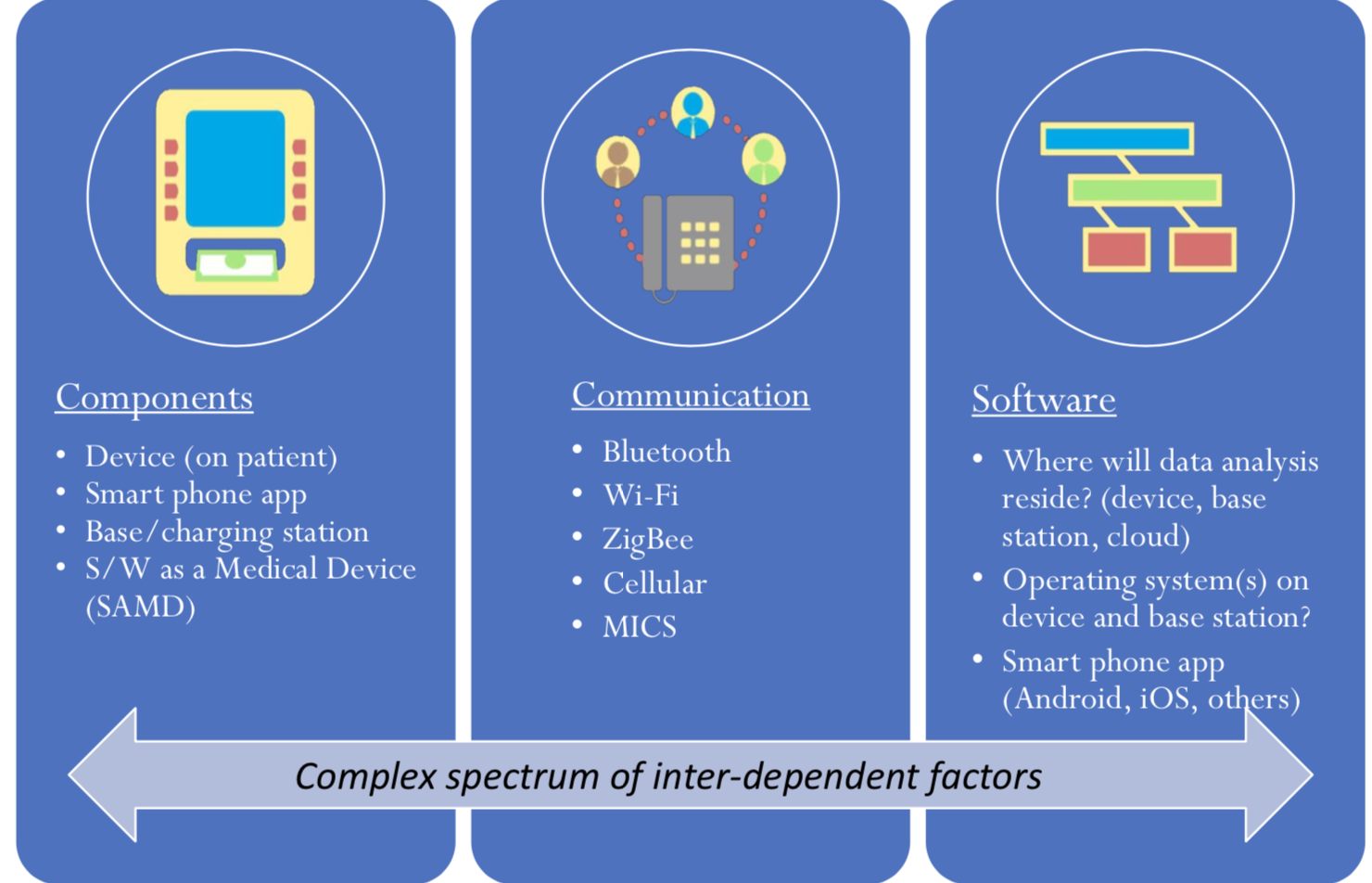
#2. What platform design options should I address?
There are several different routes you can take to create a connected device. For example, just beginning with the components of the device, will it be a wearable or implantable? Will it be “software as a medical device”, perhaps an app on a phone?
Will the device just collect data and send it somewhere else for processing? Or, will it be able to analyze that data itself? How will it communicate? For example, Bluetooth, WiFi, and MICS are options. (MICS is the Medical Implant Communication Service that has been around since the early 2000s. However, most companies we have been working with are opting for Bluetooth).
Software and data storage are key considerations, too. If you consider the medical device itself, it doesn’t usually have a lot of storage space available. On the other hand, when data is stored on the cloud it opens up virtually endless storage space.
Another important point is that stored data can be analyzed and used to help inform key decisions about the device or patient. An example is heart pumps. Predictive analytics can be used to analyze data across multiple patients and pumps in use. This could give device manufacturers the ability to pinpoint specific devices and predict when a catastrophic failure was about to happen, for example.
#3. What market opportunities should I consider?
The technology exists so that connectivity can make a huge difference in the lives of patients and physicians. For example, the current novel coronavirus pandemic has highlighted some inefficiencies that connectivity can mitigate, such as people waiting for a long period to get their test results.
The opportunities tend to lie around the regulatory environment and how this might shift to accommodate more benefits of connectivity.
Use cases
Here are some use cases that represent opportunities for connected devices:
- Reimbursements. These often hinge on being able to demonstrate device usage as part of therapy or monitoring. Proving this becomes much easier with a connected device–usage data is automatically captured and reported!
- Compliance. Devices in clinical trials must be able to demonstrate that the device was being used according to specific protocols. Again, this is much easier to prove with a connected device.
- Device diagnostics. Monitoring and diagnosing the health of the device itself is much easier with connectivity. Device parameters can be collected continuously and analyzed in real-time–alerts about battery level and other types of performance degradation can be sent automatically to the patient, provider, and the device manufacturer.
Final thoughts
What next? Consider how your medical device aligns with cloud connectivity. What technical and design issues do you have? What about the sensor platform?
With the goal of market acceptance in-mind, connectivity has become a “must-have” for new medical devices. Getting your device connected can give you a competitive advantage and present new market opportunities.
Talk to us here at Galen Data about how our fully-operational and compliant Galen Cloud™ connectivity platform can help with your needs.