AI and the FDA – What New Guidance Means for AI Innovation in Healthcare
When ChatGPT exploded on the scene in late November 2022, Artificial Intelligence (AI) became the mainstream hot topic of discussion. And for good reason; AI’s potential influence on human society is almost infinite and accelerating rapidly.
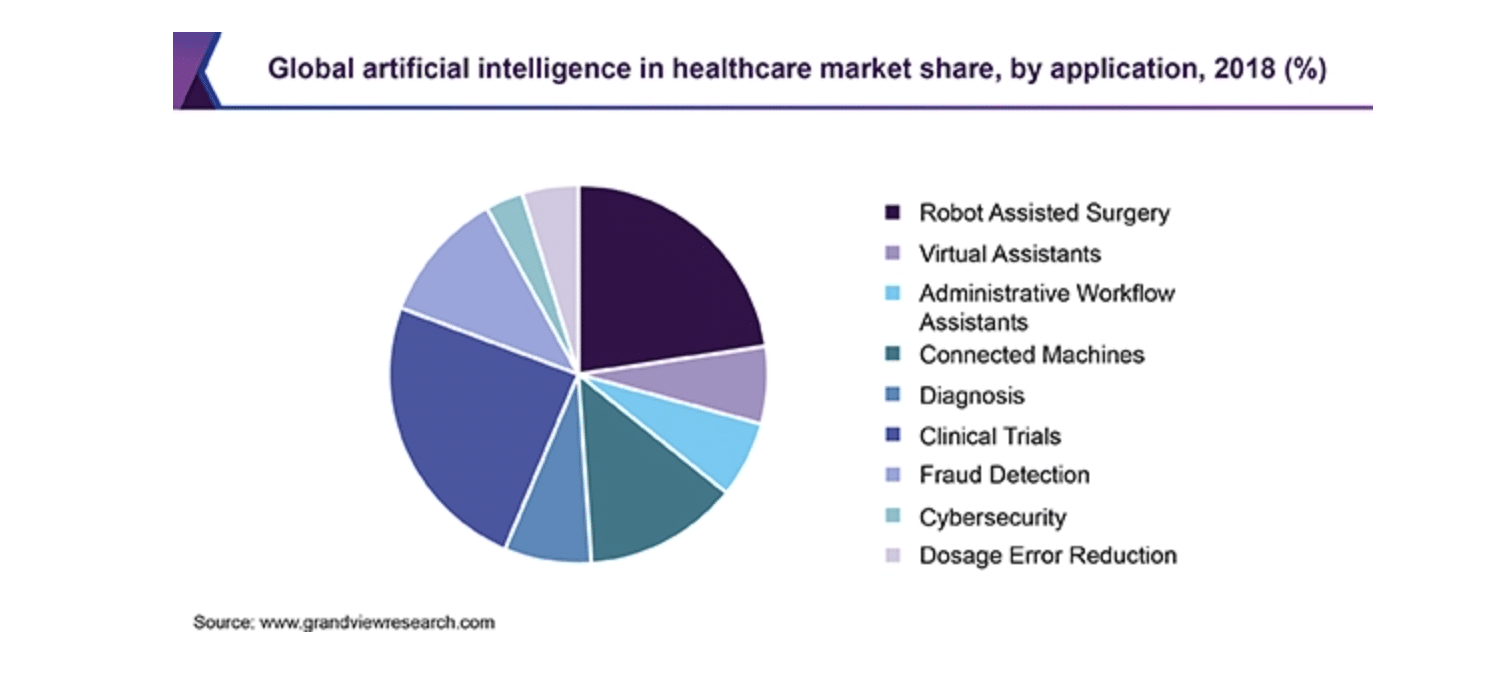
The healthcare industry is lagging slightly in AI adoption compared to other industries. Even so, connected medical devices are one sector already directly affected by AI. As early as 2018 AI was making its presence known in the robot assistant surgery and clinical trials sectors, for example.
AI has the potential to revolutionize healthcare delivery and enhance patient outcomes. However, with every miracle there is always a catch; AI for advanced medical devices is no exception. There are some downsides.
In the US, advanced medical device companies look to the FDA for perspective and guidance. The agency is the regulatory gatekeeper to the lucrative US market. Since AI will soon affect nearly every connected medical device, let’s take a closer look at AI’s influence on healthcare and the FDA’s current stance.
Benefits of AI in Advanced Medical Devices
AI algorithms can interpret vast patient data, enabling early disease detection, accurate diagnosis, and personalized treatment plans. AI’s potential benefits for advanced medical devices are hard to fully grasp, even as it expands. We’ve created an easy-to-scan list below of AI benefits to help you get an overview.
- Enhanced Data Analysis: AI enables advanced medical devices to process and analyze vast amounts of patient data quickly and accurately, leading to more precise and actionable insights for healthcare professionals.
- Personalized Treatment: AI-powered medical devices can tailor treatments based on individual patient data, including genetics, lifestyle, and medical history, providing personalized and targeted interventions for better patient outcomes.
- Early Disease Detection: AI algorithms can detect subtle patterns and anomalies in medical data, facilitating early detection of diseases and conditions and allowing for timely intervention and improved prognosis.
- Real-Time Monitoring: Advanced medical devices with AI capabilities can continuously monitor patient vital signs and health parameters in real-time, alerting healthcare providers to any concerning changes and enabling immediate responses.
- Improved Diagnosis Accuracy: AI-powered medical devices can assist in medical imaging and diagnostics, enhancing accuracy and reducing the likelihood of misdiagnosis, leading to more effective treatment planning.
- Remote Patient Management: AI-enabled medical devices support remote patient monitoring, enabling healthcare professionals to track patients’ conditions remotely, manage chronic diseases, and reduce hospital visits.
- Surgical Precision: AI-integrated surgical devices offer enhanced precision and stability during complex procedures, minimizing surgical errors, reducing recovery times, and improving overall surgical outcomes.
- Predictive Maintenance: AI can analyze device performance data to predict potential failures or malfunctions, enabling proactive maintenance, reducing downtime, and ensuring continuous device functionality.
- Workflow Efficiency: AI streamlines device operation and data processing, reducing the workload on healthcare professionals, enabling them to focus on patient care and improving overall healthcare delivery.
- Continuous Learning: AI-powered medical devices can continuously learn from new data and feedback, improving their performance and adapting to evolving medical challenges, ensuring cutting-edge capabilities over time.
Challenges with AI in Advanced Medical Devices
While the above list is impressive, there are downsides. At the most basic level, AI is actually not a new shiny object. It is an evolutionary technology based on vast amounts of data, both historical and ongoing. In health care, much of this data is highly regulated patient data.
Let’s look at a few of the challenges.
- Data Privacy and Security: One of the significant challenges in AI healthcare is ensuring the privacy and security of patient data. AI systems often require access to sensitive medical records, making them vulnerable to data breaches and unauthorized access.
- Bias and Fairness: AI algorithms can inadvertently perpetuate biases in the training data, leading to biased treatment recommendations and decisions. Ensuring fairness and avoiding discrimination is crucial to maintaining ethical AI healthcare practices.
- Lack of Interoperability: Integrating AI systems with existing healthcare infrastructure can be challenging due to the lack of standardized data formats and interoperability between healthcare IT systems.
- Regulation and Compliance: The rapidly evolving nature of AI technology poses regulatory challenges in healthcare. There is a need to establish clear guidelines and standards to ensure that AI applications meet safety and efficacy requirements.
- Ethical Use and Decision-Making: AI systems can generate complex decisions that are difficult to interpret and explain. Ensuring ethical use and accountability for AI-generated decisions is essential, particularly in critical medical scenarios.
- Limited Transparency: Many AI algorithms operate as “black boxes,” making it challenging to understand their decision-making processes. Transparency and interpretability are crucial for gaining trust in AI applications used in healthcare.
- Validation and Reliability: Validating the accuracy and reliability of AI models is crucial before integrating them into medical practice. Rigorous testing and evaluation are necessary to ensure their safety and effectiveness.
- Physician Acceptance and Training: Healthcare professionals need to understand and trust AI technology for successful integration into clinical workflows. Proper training and education are essential to ensure efficient and responsible use of AI tools.
- Data Quality and Bias Mitigation: AI algorithms heavily rely on high-quality and representative data for optimal performance. Ensuring data quality and mitigating bias in the training datasets are critical challenges.
- Cost and Resource Allocation: Implementing AI in healthcare may require substantial investments in infrastructure, staff training, and ongoing maintenance. Balancing costs and allocating resources effectively is crucial for widespread adoption.
With this overview of the pros and cons, we can better understand the balance the FDA is trying to strike in regulating AI/ML in medical devices. Let’s look at that next.
FDA Guidance on AI in Advanced Medical Devices
Overall, the FDA’s proposed approach aims to accelerate medical device innovation and enable more personalized medicine. Still, developers will need to navigate the complexities and documentation requirements to benefit from the potential improvements in AI/ML-enabled devices.
In 2021, the FDA released draft guidance proposing a science-based approach to requirements for medical devices powered by AI and machine learning (ML). The goals of the guidance include enabling safe and rapid modification, updating, and improvement of AI/ML-enabled devices in response to new data.
The guidance includes a Predetermined Change Control Plan (PCCP) that companies must agree upon with the FDA. This PCCP scope includes:
- A detailed description of the device modifications
- The methodology for testing and implementing these changes.
- An assessment of the benefits and risks of the modifications.
- How they plan to inform users of the changes.
The FDA emphasizes that these control plans apply to AI/ML-enabled software as medical devices and to all AI/ML-enabled device software functions. As of late July 2023, the current list of AI/ML devices on the FDA’s website includes 521 entries.
FDA leadership believes that the PCCP helps in continuously improving AI/ML-enabled device performance across diverse populations, while also addressing essential considerations such as race, ethnicity, disease severity, gender, age, and geographical factors.
The FDA draft guidance is part of a larger trend in healthcare of building confidence in AI/ML models. Share on XRegulation and compliance are crucial to building trust in AI’s impact on clinical decisions.
What does FDA Guidance on AI mean for Advanced Medical Device Teams?
While the PCCP guidance seems straightforward enough, legal experts advise medical device developers to prepare for some challenges. The documentation requirements going forward will scale significantly, similar to those required for submissions. Developers will need to keep all the documentation in their files if the FDA decides to inspect their work.
FDA compliance with AI considerations is going to have a major impact on medical device companies of all sizes and stages. At Galen, we have years of experience navigating the evolution of advanced medical device regulation. Reach out today if you have any questions about how AI and FDA compliance may affect your advanced medical device business.