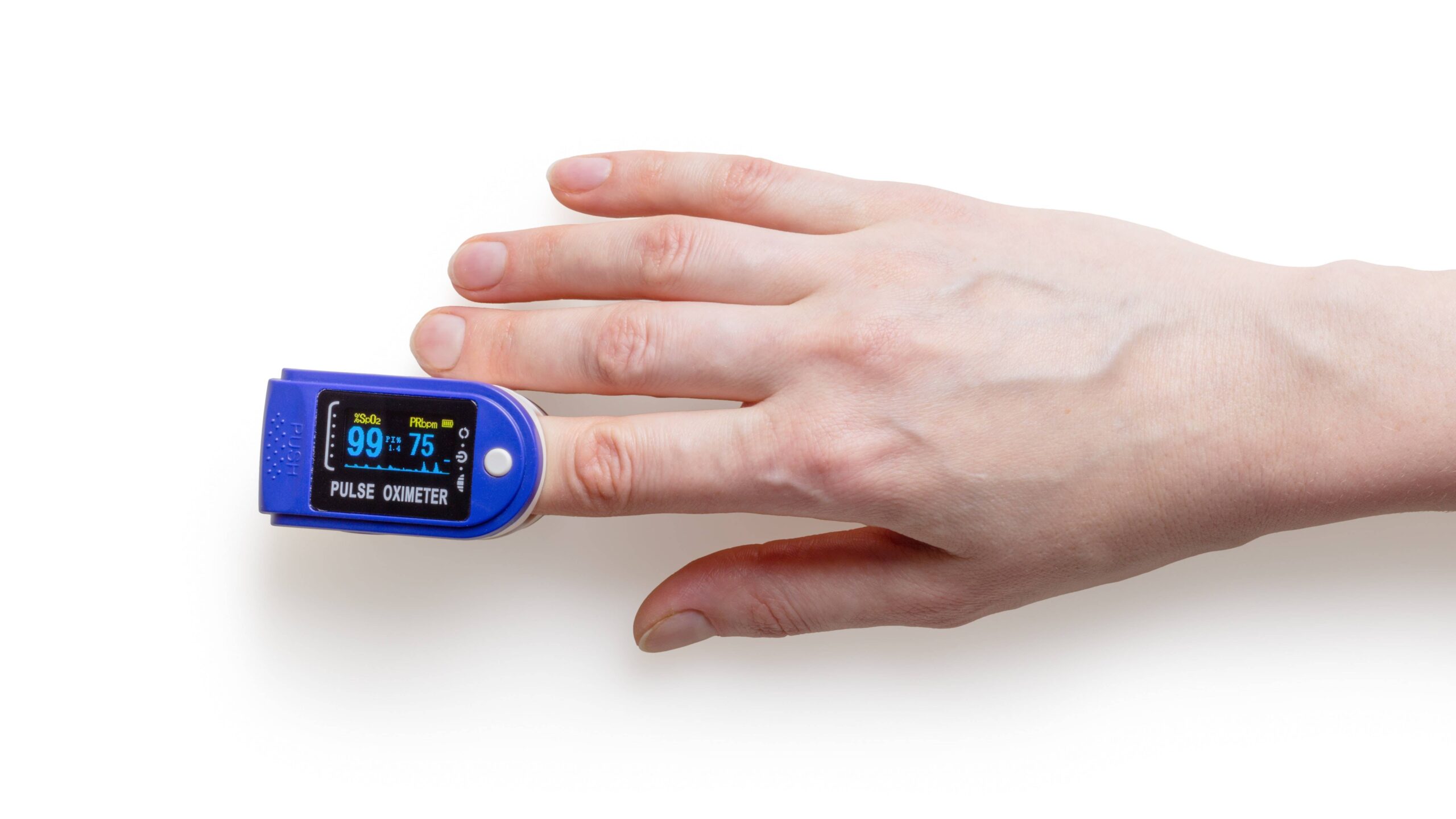
Managing Downside Risks After Launch – Post Market Best Practices for Connected Medical Devices
When advanced medical device (AMD) startups are in the thick of device design and development, getting to market is the light at the end of the tunnel. While Go To Market (GTM) approval is a huge milestone, a market launch is simply the start of an even longer marathon that doesn’t end until the device […]