3 Top Trends in 2023 for Medical Device Startups
The advanced medical device industry is inseparable from trends in technology and data, two sectors of exponential business growth in recent years. In healthcare, the global pandemic accelerated demand in certain segments like telehealth, service robots, and remote monitoring.
This year the world continued to adjust to the aftermath of COVID-19 and mounting economic uncertainty. Even with workforce challenges, healthcare and related technology continue to be one of the more resilient sectors. Let’s look at three of the top tech-fueled trends for medical devices in 2023 – telemedicine, personalized medicine, and wearable devices.
Even with workforce challenges, healthcare and related technology continue to be one of the more resilient sectors. Share on XTelemedicine Takes Off
A study by Global Industry Analysts valued the global telemedicine market at US$ 68.36 billion in 2021 and predicted a compound annual growth rate (CAGR) of 22.3% from 2022 to 2029, reaching nearly US$ 342.17 billion.
Factors influencing industry growth include increased broadband and mobile network penetration. Stakeholders interested in country or regional demand can track or perhaps even predict demand curves for telemedicine services by following major broadband installation and improvement projects in 2023.
High costs in healthcare are also creating a growing demand for cost-effective and convenient healthcare delivery models. Since the global pandemic, patients and consumers have become more comfortable with online meetings. Telemedicine can provide a suitable alternative for many provider visits that used to happen in an office.
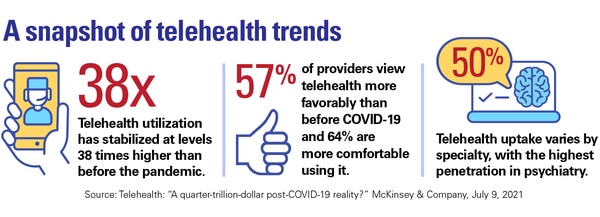
One interesting point for medical device startups is that telehealth usage varies widely by specialty. The highest penetration is in psychiatry (50%) and substance-use treatment (30%). This could have cross-over implications for niching medical device wearables, for example.
Dimensions of Telemedicine
One dimension of telehealth centers on the administration of communication logistics. Large consumer companies like ATT and Amazon now have telehealth services for communications or documentation technologies. Healthcare native companies also offer administrative coordination services such as video conferencing, EHR integration, patient portals, and schedulers.
In connected devices, all data is not considered equal. Health data are subject to strict HIPAA privacy regulations and sharing restrictions. Cybersecurity and compliance are inseparable from the design and implementation of telehealth networks.
The FDA clearly states that medical device manufacturers and healthcare delivery organizations are responsible for data security.
Personalized Medicine and Treatments
In the early 2000s, biologist Leroy Hood introduced a patient-centered decision system called the 4Ps – describing a process where healthcare would evolve to be more predictive, preventive, personalized, and participative. Today, we can see many aspects of that theory becoming a reality.
Providers can now combine individual patient data and medical history with AI-sourced predictive analytics about trends and probabilities. Combining personal and analyzed data helps providers identify which patients are most likely to experience a particular outcome and customize their treatment accordingly.
The “wait and see” time to observe how patients adjust to treatments is also shrinking. Medical devices provide real-time feedback on how a patient is responding to treatment. Devices detect red flags in patient responses, sometimes even before the patient is aware of a potential problem. Doctors can intervene and adjust the treatment plan to achieve better outcomes sooner.
As promising as many of the predictive analytics results are, medical startups interested in the evolving use of AI should be aware of potential risks. Data bias can skew results for specific patient groups. Medical device manufacturers should track ongoing developments to mitigate potential bias in data sets.
The Boom in the Wearable Devices
According to recent projections, the global wearable healthcare devices market will increase by 13.2% from USD 16.2 billion in 2021 to USD$ 30.1 billion by 2026.
While some wearables target patient applications, others are mostly for consumer use. From a regulatory standpoint, there is a clear line between wearables for patient use and consumer wearables that track non-invasive basic metrics.
In 2023, we will see an increase in what some are calling a medical-grade wearable device that combines the capabilities of both medical and consumer-grade devices.
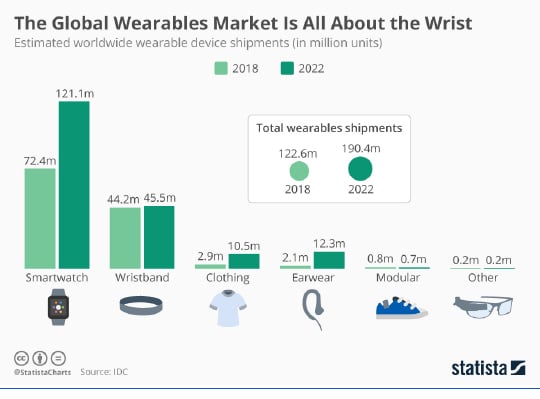
Up to this point, the largest form factor category for the consumer market is in wrist accessories, specifically the smartwatch. It’s not clear, though, how much of the smartwatch purchases volume is driven by the desire for health and fitness monitoring.
Unfortunately, the world is seeing an increase in lifestyle diseases. Older adults ages 51 to 61 had a higher prevalence of six out of eight chronic conditions in 2004-2010 than their peers in 1992-1998, according to a 2016 study. Obesity and diabetes are increasing in the US and globally.
Trends driving the demand for wearables include:
- Consumers are more connected to information and each other than ever. Consumer wearables appeal to health-conscious people who are actively interested in health and wellness.
- Increasing rates of lifestyle diseases.
- Rising healthcare costs, even as lifestyle disease diagnoses increase, have providers looking for affordable ways to manage chronic conditions.
- Population trends with generational demand for fitness and wellness services and gadgets from large demographics, especially millennials. For aging Americans, Medicare Advantage now covers wearable devices that monitor heart health and physical activity.
In 2023, regulated medical device wearable startups and other stakeholders should monitor how quickly the insurance industry, including Medicare, is moving to cover additional wearable devices based on personalization and preventive benefits.
Moving Ahead
When developing a medical device roadmap, it can be helpful to try to get ahead of the more significant industry trends. We have an informed perspective based on years of experience working with a wide range of startups. How might 2023 affect your medical device startup? Feel free to reach out today to get the conversation started.






