How to Choose a Product Development Team Partner
Taking an advanced medical device from concept to successful market penetration involves conquering some unique complexity. Experts often attribute the high failure rate for advanced medical device startups to discrete causes like team incompetency, testing snafus, insufficient funding, or poor-quality materials.
“The truth is that advanced medical device startup failure is often rooted in one of the overriding challenges for go-to-market success – managing complexity.” For advanced medical devices, prototyping, testing, and keeping an eye on the regulatory compliance process add to the already significant challenges in a typical startup ecosystem.
Complexity is often native to the devices themselves, too. advanced medical devices bring innovative technology to the marketplace. Instead of just another widget, innovative tech introduces the need for end-user education and new manufacturing outputs.
To manage this complexity and access expertise, advanced medical device companies often reach out to Product Development Team Partners (PDTP) at different phases of development, such as:
- Device discovery and risk analysis
- Formulation, concept, and feasibility
- Design and development – verification, validation, regulatory issues
- Final validation and product launch prep
- Production, market introduction, and post-market follow-up
As we’ll see in the next section, the five phases above are like a string of icebergs that only tell the top-line story.
The bottom line is that no matter how promising the advanced medical device technology is, time and money are limited. Success in getting to market boils down to one thing – navigating the icebergs with the superlative project management of many stakeholders.
Luckily for advanced medical device startups, there are many experienced captains in the medical device industry to help startups avoid a Titanic outcome. The right PDTP can help save money and move from concept to market on schedule.
Some of the areas of potential partnership include:
- Design and development
- Quality, regulatory, and compliance
- Contract research organization
- Contract manufacturing
- Data analytics
- Electronic data capture solutions
- Cloud connectivity
- Industry connections for business building
In this post, we’ll overview what PTDPs do and how to choose the proper support for your process.
How Does a Product Development Team Partner Help?
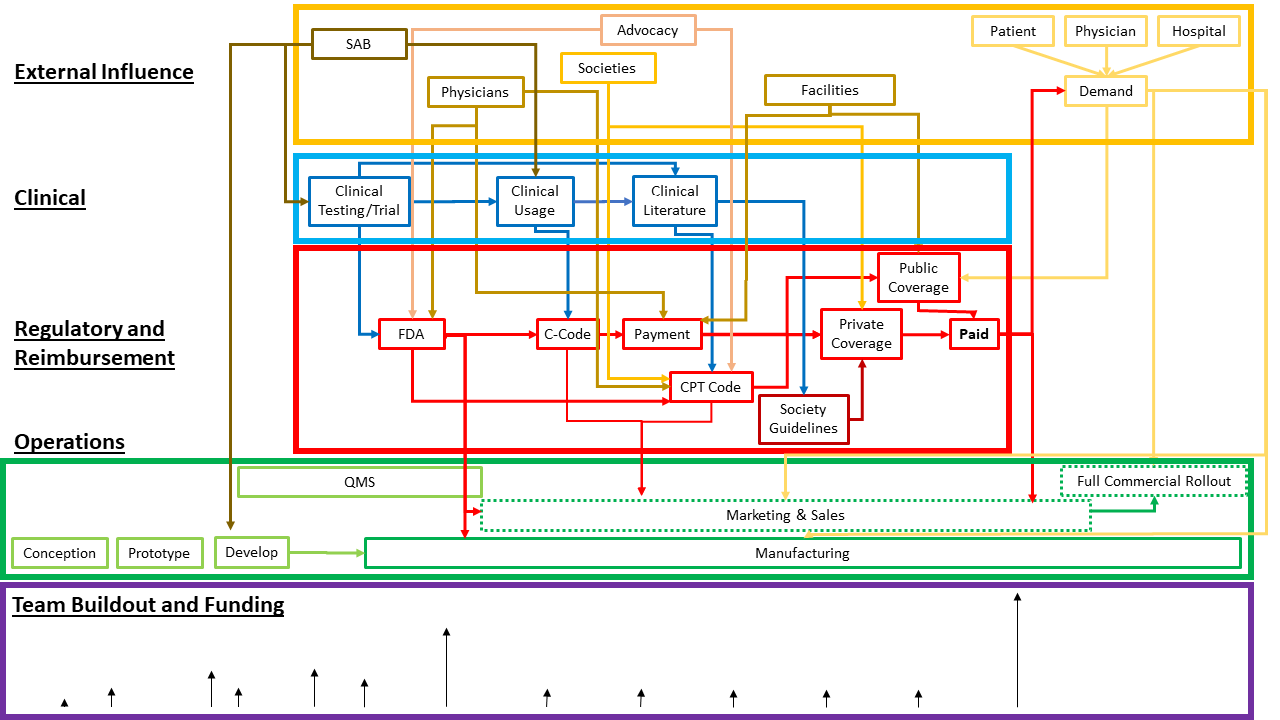
As we’ve seen, managing complexity is a crucial component of successful advanced medical device development. The visual below may vary given project specifics, yet it provides a general look at what’s below the tip of the iceberg of advanced medical device phases. Each box represents many additional moving pieces that require planning, coordination, and communication.
Each phase requires integrating new expertise while simultaneously scaling teams and other stakeholders. While it may seem simple enough to hire the right person or team for the job at the right time, in reality, integrating new people and teams adds complexity and is time-consuming.
A PDTP is a resource that companies contract to help coordinate and perform crucial aspects of the development phases. PDTPs include:
- Individual consultants and groups
- Design and development companies
- Integrated design to manufacturing partners
- Contract manufacturers
Brooks’ law tells us that due to ramp-up time and expanding communication needs, there is an incremental dip in productivity with each new person added. If management has systems in place to help with the overall flow, progress is cohesive and aligned. Without effective project management, progress stalls, and teams fall out of alignment.
Minimizing the adverse effects of Brooks’ law is why some companies opt to work with a single PDTP with experience across all the phases of development to coordinate consultants, service providers, regulatory milestones, and manufacturing stakeholders. In addition, consultant firms maintain an ecosystem of partners to help and refer to their clients.
Let’s look at a few tips on how to find the optimal PDTP for your project.
Finding the Best PDTP for Advanced Medical Device Projects
The good news is an ecosystem of service providers has grown along with the advanced medical device industry. The key is to take an organized team approach to choose the right PDTP.
Before looking for PDTPs, meet with key internal team members and develop a list of “must have” and “nice to have” qualities you need in a PDTP for your project.
Secondly, compile a list of prospects to contact. Start with who you know to exploit the referral effect. Coordinate with your team members to ask for referrals to PDTPs from trusted advisors and other contacts who understand your concept. In the interview process, you can then ask those referrals about their biggest competition and talk to those companies, too.
Finally, searching for information online will lead you to many PDTP blogs and websites. If your technology or phase requires specific expertise, searching on that keyword should help you zero in on the right potential match to add to the RFP list.
Interviewing and Choosing a Medical Device PDTP
Advanced medical devices have long product development cycles and that means you will be working closely with your PDTPs for a long time. The team you choose will have a significant impact on the success of your project, so it’s essential to take the time to interview potential partners and select the one that’s the best fit for your needs.
Consider preparing a Request for Proposal to streamline your interviewing and hiring process. The obvious starting place in an RFP is the experience of the team and their track record in developing similar products.
Secondly, do you need PDTPs that are very specialized, or do you need those that can be multipurpose with related functions? For example, do they offer an understanding of medical processes and compliance, along with turnkey engineering expertise?
Here are some more possible areas to develop a list of questions to populate your RFP:
- Track record of design relative to your concept
- Engineering expertise in engineering
- Expertise in planning for and integrating regulatory milestones
- Materials expertise in materials
- Manufacturing expertise
- Proven product development process
- Intellectual property protection safeguards
- Track record and system to align with cost goals
- Track record in meeting quality assurance standards
- Post-market release follow-up and monitoring
The final factor in choosing a PDTP is the equivalent of a “gut check”. If your startup is at the point where it is ready to scale with a PDTP, chances are the existing internal teams have achieved their own culture and flow. Ask a few team members to meet with your final candidates and provide feedback. Your PDTP needs to mesh well with your team, or communication will suffer.
Moving Ahead
Are you preparing to add a Product Development Team Partner to your project? If so, you probably have questions. We are here to help. Contact us today for an overview of our Medical Device Partner Ecosystem that combines expertise to meet a wide range of medical device company needs. Our relationships with these leading organizations create a comprehensive suite of proficiencies to contribute to your success.