Smart Planning for Growth: Data Management Scalability for Medical Devices
Advancements in artificial intelligence, the Internet of Things (IoT), and big data analytics are transforming healthcare and the medical device industry with a stream of groundbreaking innovations.
However, innovation and market success always come at a price. Data storage in the healthcare industry is becoming increasingly complex. Data management scalability for medical devices is now an essential element of success.
All these new devices are exponentially increasing the amount of sensitive data that medical device manufacturers are responsible for securing and managing.
However, innovation and market success always come at a price. Data storage in the healthcare industry is becoming increasingly complex. Click To TweetWhat is Data in Healthcare?
Healthcare data is any data related to health conditions, quality of life, and health outcomes for an individual or population. It comes from many sources, with medical devices being one of the major sources of healthcare data. From connected devices to Software as a Medical device, (SaMDs), their number one output is data.
And the growth continues. Acumen Research and Consulting reports the global connected medical devices market size is estimated to grow a CAGR above 22% over the forecast timeframe and reach a market value of US$ 181.9 billion by 2030.
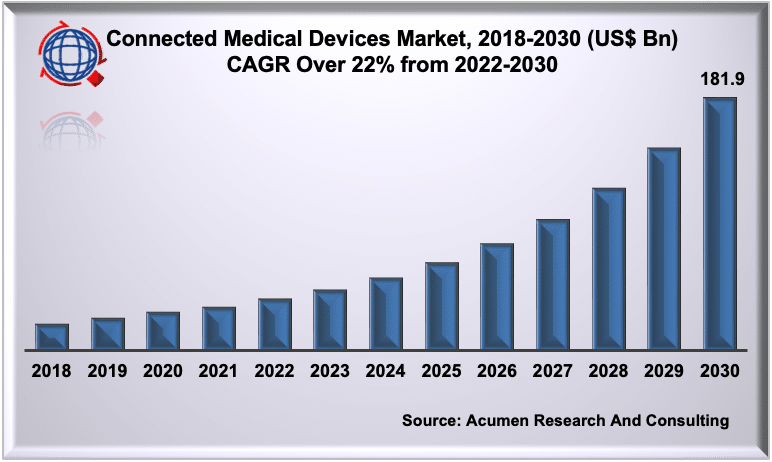
Source: Acumen Research and Consulting
Managing the increasing volume of health data is a huge challenge for the industry. Health data managers may struggle with limited storage capacity and siloed systems, making it difficult to collect, analyze, and utilize data efficiently. Security is also job one for data governance, regulatory compliance, and data storage in healthcare.
The good news is that technology is also helping with better solutions for health data management challenges. Database management in healthcare is undergoing its own “smart technology” transformation with software development, machine learning (ML), and cloud-based solutions.
At Galen Data, we were among the first to adopt a cloud-based approach. In this post, we’re sharing some of what we’ve learned. Let’s take a closer look.
Why Does Medical Device Scalability Matter?
Imagine this: your brilliant medical device has taken the market by storm. Doctors love it, you are seeing improved patient outcomes, healthcare organizations are calling, and your company is experiencing phenomenal growth. Even the VCs are happy.
But amidst the celebration, a crisis is brewing. The sheer volume of data your device generates threatens to overwhelm the existing system. The nurses aren’t happy. They are juggling multiple electronic health records (EHR) systems. The overload of data management in nursing frustrates staff and leads to inconsistencies.
This scenario, while hypothetical, reflects a real challenge facing companies today. As devices become more sophisticated and interconnected, the amount of data they produce grows exponentially.
Traditional storage solutions simply can’t handle this “data deluge.”
And there’s more. The data challenge doesn’t arise only in the marketplace. The strategic approach to data scalability starts at the very beginning of product development.
The Data Scalability Challenge: From Pre-Clearance to Post-Sales
The data scalability challenge for connected medical devices is dynamic, evolving from research design to GTM and beyond. Here’s a breakdown of the specific hurdles you’ll encounter at each stage of the development process, from research to pre-clearance to post-sales in the marketplace:
Research and Development
Data management in health research is the foundation for medical progress. Researchers rely on robust data collection, storage, and analysis to identify trends, develop new treatments, and ultimately improve patient care.
Pre-Clearance Data Strategy:
Prototype Stage: During the initial development phase, data collection may be limited. However, it’s crucial to establish data best practices from the outset. Choose a cloud platform that offers secure storage for design files, test results, and user feedback. Utilize tools to categorize and organize data effectively, ensuring easy retrieval and analysis in the future.
Limited Data, High Scrutiny: During the early stages of device development, data collection might be relatively modest. However, the data quality is crucial for regulatory requirements and pre-clearance. The challenge here lies in ensuring the structure and security of this initial data.
Scaling Up: Clinical trials represent a significant leap in data volume. You’ll collect patient records, device usage logs, and performance metrics on a much larger scale. This sudden influx of data can overwhelm a platform not designed for scalability.
Here, the focus shifts to secure storage and efficient management. Your cloud platform must be HIPAA-compliant to protect health records and patient privacy and offer robust access controls to ensure that only authorized personnel can access sensitive trial data. Also, consider features like automated data anonymization to safeguard patient information.
Data Scalability in Medical Device Post-Market Surveillance and Sales
The Data Deluge: Once your device hits the market, the data floodgates open. Real-time usage data, anonymized patient information, and detailed performance metrics will pour in continuously. This is where the true test of scalability comes in. Look for features like automatic storage and processing power scaling to ensure smooth operation even as data volumes surge.
Data Variety and Integration: Connected devices may generate a variety of data formats, including text logs, sensor readings, and even video or audio recordings. The challenge is integrating diverse data into a cohesive and analyzable format. A scalable cloud platform should offer data integration tools and data analysis capabilities to handle this heterogeneity, allowing you to extract valuable insights from all the data your devices generate.
Long-Term Storage and Compliance: Regulatory agencies often mandate the retention of medical device data for several years. This necessitates scalable storage and archiving capabilities that ensure long-term data integrity and compliance. The platform should offer secure, cost-effective archiving solutions that meet industry regulations, such as FDA’s 21 CFR Part 11.
By understanding the challenges at each stage of the development process, you can choose a cloud platform that can grow alongside your device.
How the Cloud Benefits Data Management Scalability for Medical Devices
Cloud-based data platforms offer game-changing data management benefits. Unlike traditional on-premise solutions that require significant upfront investments in hardware and software, cloud platforms provide a more flexible and cost-effective alternative.
Here’s how cloud-based platforms like Galen Data empower medical device companies to achieve scalability:
Flexible Storage Options: Cloud storage allows you to scale your data capacity seamlessly as your needs evolve. You only pay for the resources you use, eliminating the need for expensive upfront investments or the risk of underestimating future storage needs. This is particularly beneficial during the early stages of development, where data volume is likely to be lower.
Enhanced Data Security: Cloud platforms offer robust security features, including encryption, controlled access, and regular security audits. This ensures sensitive medical data is protected from unauthorized access, data breaches, and cyber threats. Additionally, leading cloud providers invest heavily in state-of-the-art security infrastructure, offering a higher level of protection compared to in-house solutions for many companies.
Efficient Handling of Increased Data Volumes: Cloud platforms are built to handle massive datasets. They are designed to automatically scale storage and processing power as needed, ensuring seamless performance even when data volumes surge. This eliminates the need for constant hardware upgrades and IT resource allocation, allowing you to focus on core business activities.
Improved Collaboration: Cloud platforms facilitate collaboration between geographically dispersed teams. Secure data exchange is readily accessible from anywhere with an internet connection, allowing engineers, researchers, and regulatory teams to work together seamlessly, regardless of location.
Disaster Recovery and Business Continuity: Cloud platforms offer built-in disaster recovery features that ensure data remains accessible even in the event of a hardware failure or natural disaster. This minimizes downtime and ensures your device development efforts can continue uninterrupted.
Moving Ahead with Data Management Scalability for Medical Devices
As technology fuels innovation and new solutions, it’s leaving a flood of data in its wake. Are you working on a medical device? Planning for data storage and security isn’t just about scaling – it’s about building a foundation for success from the beginning.
At Galen Data, we understand the unique challenges of medical device data and have the cloud expertise to help you navigate them.
Partner with Galen Data today to:
- Develop a secure and scalable data management plan.
- Leverage our expertise in medical device data and compliance.
- Focus on innovation while we handle the infrastructure.
Schedule a call with us today to discuss your specific needs and see how Galen Data can help you store, manage, and secure your medical device data at scale.