Spotlight on Innovation in Pharmatech and Medical Devices
Pharmatech, a cutting-edge field at the intersection of the pharmaceutical industry and advanced technology, is rapidly transforming healthcare by leveraging cutting-edge technologies for enhanced drug discovery and development. The synergy between Pharmatech and medical devices is growing.
Advanced Medical Devices play a critical role, not just as tools for pharmaceutical delivery but as active partners in US Pharmatech and global growth. The fusion of Pharmatech and medical devices integrates sophisticated software and hardware to support complex pharmaceutical applications.
Key Technologies Driving Pharmatech Advancements
Several key technologies are driving Pharmatech’s evolution. Artificial Intelligence (AI) and Machine Learning (ML) are at the forefront, significantly accelerating the pace of drug discovery by predicting potential drug candidates and modeling their interactions with biological targets.
In drug development, Big Data analytics and cloud computing provide robust platforms for managing vast amounts of clinical data, enhancing the accuracy and speed of trial outcomes. Furthermore, wearable advanced medical devices and the Internet of Medical Things (IoMT) are revolutionizing patient monitoring, data collection, and personalized medicine approaches.
The Intersection of Pharmatech and Medical Devices
The difference between Pharmatech and medical devices lies mainly in focus. Understanding their differences can help you see how they complement each other in modern healthcare. Here are some differences between Pharmatech and medical devices:
- Focus: Pharmatech encompasses digital tools, software, and advanced technologies in drug discovery, development, and distribution. Pharmatech aims to enhance pharmaceutical research and production efficiency, speed, and effectiveness.
- Technologies Involved: In Pharmatech, technologies such as Artificial Intelligence (AI), Machine Learning (ML), Big Data, and cloud computing play significant roles. These technologies are employed for analyzing biochemical interactions, predicting drug efficacy, and managing research data.
- Regulatory Aspects: The regulatory considerations for Pharmatech mainly focus on drug safety, efficacy, and quality control. This includes adherence to Good Manufacturing Practices (GMP) and regulations of clinical trials, drug approvals, and post-marketing surveillance.
- End Products: The end products of Pharmatech incorporate some aspect of pharmaceutical drugs and therapies, including new molecular entities, generics, and biological
Examples of Pharmatech in Advanced Medical Devices
Pharmtech influences device development by closely tying device functionality to pharmaceutical outcomes. Modern advanced medical devices are often embedded with sensors and connected technologies, enabling them to collect real-time patient data. This data allows for personalized drug therapies and continuous monitoring in clinical trials. Here are some examples of Pharmatech in advanced medical devices:
Smart Inhalers
These are advanced inhalers equipped with digital technology to improve medication adherence in respiratory conditions like asthma and COPD (Chronic Obstructive Pulmonary Disease). They track usage, remind patients to take their medication, and can even provide data to healthcare providers about the patient’s inhaler use patterns.
Wearable Drug Delivery in Pharmatech and Medical Devices
These include devices like insulin pumps for diabetes management, which can be programmed to deliver precise insulin doses according to the patient’s needs. Some of these pumps are becoming increasingly sophisticated, integrating continuous glucose monitoring and automated insulin delivery based on real-time glucose levels.
Digital Pills
These are a novel pharmaceutical product that combines medications with ingestible sensors. Once swallowed and in the stomach, the sensor transmits data to a wearable patch or smartphone app, allowing for tracking of medication adherence and physiological responses to the medication.
AI-Driven Drug Discovery Platforms
Companies are utilizing AI and ML algorithms to analyze vast amounts of biological data to identify potential drug candidates more quickly than traditional methods. This approach speeds up the drug discovery phase and can identify novel therapeutic targets.
3D Printed Drug Delivery Devices:
Companies are using advanced 3D printing technology to create personalized drug delivery devices and custom dosages of medications tailored to the specific needs of individual patients.
The bottom line is that the intersection between Pharmatech and advanced medical devices creates increasingly digital, personalized, and efficient healthcare options.
Investor Questions about Pharmatech Advanced Medical Devices
Most medical device startups rely on investor funding. With global economic uncertainty, private equity is tightening its belt, and the Pharmatec sector along with many others is feeling the effect. US pharmaceutical industry private equity deal volume decreased by 48% in Q3 2023 versus Q2 and was 54% lower than last year’s Q3.
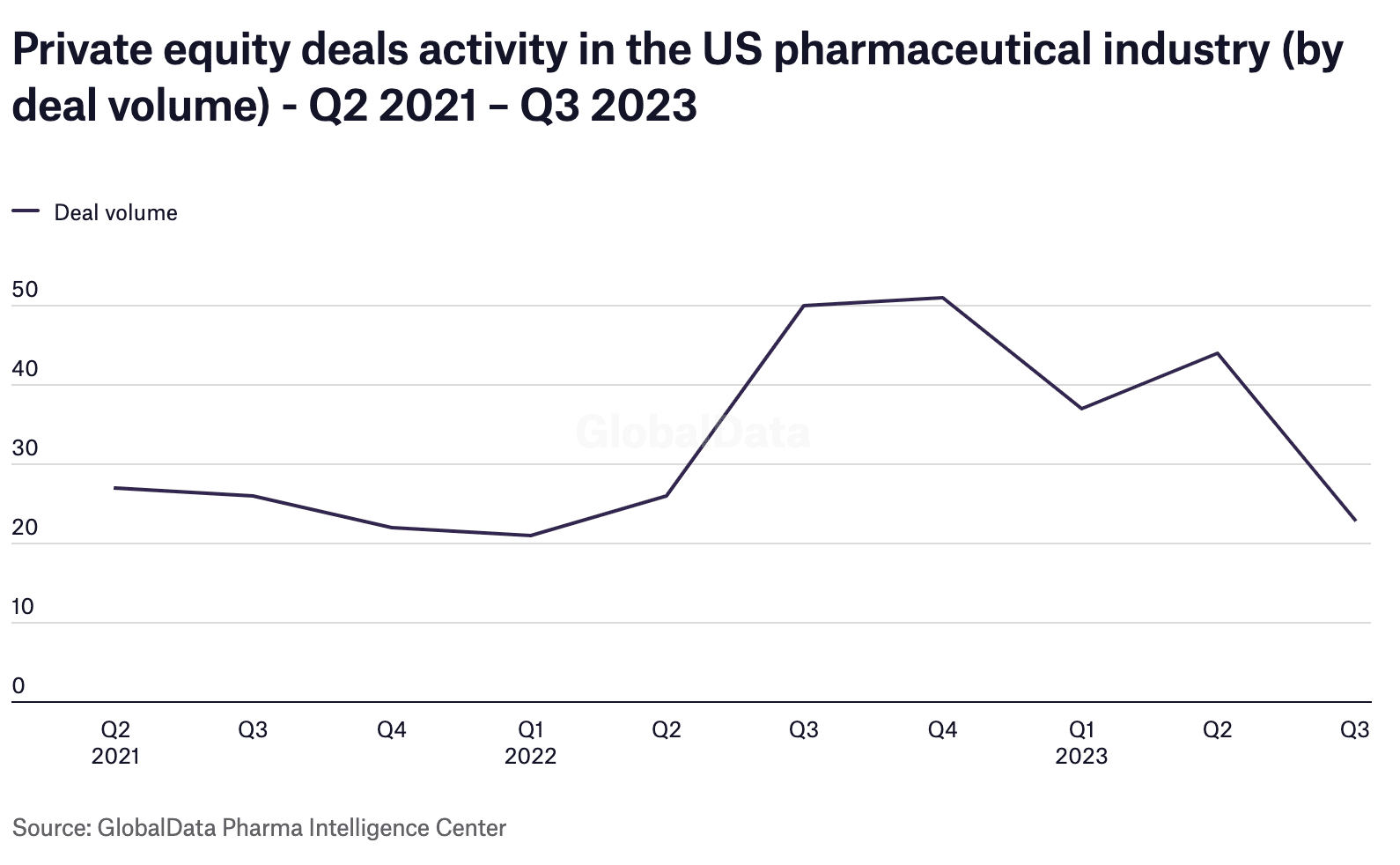
Obviously, the funding landscape is more competitive than ever. Taking stock of what is critical to investors is an essential pre-planning exercise for Pharmatech startups. In addition to traditional questions about technology, market size, and research, Questions investors ask about projects with AI include:
- Does your AI algorithm involve any IP?
- How do you source annotated data in the near term and at scale?
- Who owns the data?
- How often would you need to tune your algorithm, and what are the costs/effort each time?
Regulatory Landscape for Pharmatech Industries and Advanced Medical Devices
Alongside investment, regulation is another crucial hurdle for advanced medical device projects. The regulatory landscape for Pharmatech and advanced medical device is evolving to keep pace with technological advancements.
Regulatory bodies like the FDA are implementing frameworks that accommodate the unique challenges posed by digital health solutions and AI-driven devices.
Considerations that the FDA pays attention to include locked vs. adaptive algorithms, data quality, data privacy, cybersecurity, and the validation of AI algorithms used in drug discovery and advanced medical devices.
Staying current with regulatory changes is critical for companies to ensure compliance and market success. You can check out Galen Data’s article here for an overview of the FDA’s stance on AI and medical device development.
Future Trends and Impact of Pharmatech and Advanced Medical Devices
The future trajectory of Pharmatech and advanced medical device points towards an increasingly integrated and patient-centered healthcare system. Trends like telemedicine, remote patient monitoring, and AI-enabled diagnostic tools are gaining momentum.
Nanotechnology in medical devices and drug delivery systems is an emerging field with huge potential. Nanoparticles and nanocarriers deliver drugs to specific sites in the body, reducing side effects and improving treatment efficacy. For example, nanoscale-based pharmaceuticals are emerging to provide new and innovative drugs to overcome the limitations of traditional cancer therapy.
COVID-19 highlighted supply chain weaknesses in the healthcare system. Industry leaders are turning to blockchain for secure and transparent drug supply chains.
Aging populations and sedentary lifestyles in developed countries will also drive demand for Pharmatec products, creating more opportunities for Pharmatec jobs and Pharmatech associates training in Pharmatech courses.
Moving Ahead with Pharmatech and Advanced Medical Device
Are you developing an innovative Pharmatech advanced medical device? The cutting edge of Pharmatech is exciting, yet its intersection with the real world means more complexity in the funding and FDA approval journeys.
You don’t have to go it alone. At Galen Data we have extensive experience helping medical device companies navigate both the funding and the regulatory labyrinth. We’re here to help.
Reach out today, and let’s discuss the steps toward turning your innovative vision into a market-ready solution.






