Unraveling the Complexity of Medical Device Development Costs: Why Is It So Expensive and Time-Consuming?
Anyone in advanced medical device development can tell you that the process is a complex moving target. Advanced medical devices incorporate the cutting edge of innovation, often treading on unproven ground. Budgets and timelines are challenging to predict.
However, there is an underlying template for the development process. This article explores the different stages and how teams can mitigate risks, medical device development costs, and timelines.
What is Medical Device Development?
While this may seem like an obvious question, it’s worth reinforcing the fact that advanced medical device development is a highly complex process involving dozens of stakeholders and an incredible amount of detailed documentation. Managing complexity is the key to controlling costs and meeting milestones.
The good news is that startup teams don’t have to reinvent the wheel when it comes to navigating medical device development. The basic stages are ideation, design, testing, regulatory compliance, and finally, market launch.
The time it takes to develop a medical device varies widely, ranging from a few years to over a decade, depending on the device’s complexity, the necessary research and development (R&D), and the regulatory hurdles involved.
Strategic pre-planning and efficiently managing the complexity can save time and money. There are now robust planning tools and consultants with deep experience in the comprehensive process of bringing a medical device from an idea to a market-ready product.
Preventive Actions to Control Costs Throughout the Advanced Medical Device Development Lifecycle
It’s hard to overstate the importance of the pre-planning phase in controlling advanced medical device development costs. Early milestones can help advanced medical device teams avoid costly missteps down the line. Here are some global considerations for managing costs and schedules during advanced medical device development:
It's hard to overstate the importance of the pre-planning phase in controlling advanced medical device development costs. Click To TweetProject Management Excellence: Utilize effective project management tools and techniques to keep the project on schedule and within budget. Software as a Medical Device (SaMD) projects, in particular, require careful coordination between software developers, medical professionals, and regulatory experts to align development timelines with industry standards. Galen was an early pioneer in helping SaMD teams, if you have questions, we are here to help.
Stakeholder Communication: Maintain clear and consistent communication with all stakeholders, including team members, investors, and regulatory bodies.
Agility and Adaptability: Be prepared to adapt to new information, changes in the regulatory landscape, and evolving market needs.
Implementing these preventive actions can help medical device development teams navigate the complex process more efficiently, reducing the likelihood of costly delays and budget overruns.
Funding: Working with Investors and Funding Rounds
Securing funding is a multi-stage process tied to device development milestones. It’s essential to have a funding strategy that maps to your advanced medical device development timeline.
Working with investors and navigating funding rounds influences the timeline and costs of development. For starters, investors like to see a well-defined device concept and a clear path to FDA approval or CE Mark certification.
Traction with stakeholder relationships, research and feedback from prospective end-users, and clear demonstration of need and demand in the marketplace are all positives from the investor perspective. Having a robust product development plan can attract the right investors and expedite funding.
What Are the Medical Device Development Costs and Timeline Issues in Each Phase?
The development of medical devices is heavily influenced by the need for innovation, safety, and compliance with regulatory standards. Understanding each phase’s impact on time and cost is essential for developers to navigate this challenging landscape effectively.
1. Device Discovery and Risk Analysis
In the discovery phase, developers conduct thorough research to identify market needs and analyze potential risks associated with the device. R&D challenges in this phase significantly impact the development timeline and costs.
Streamlining research processes and employing strategic risk analysis can save time and money, setting a solid foundation for subsequent stages of advanced medical device development.
Considerations for this phase include:
Conduct Thorough Market Research: Understand market needs and gaps to ensure the device meets actual demand. This is particularly crucial for Software as a Medical Device (SaMD)s, because understanding the specific software needs and integration capabilities in a given sector may influence the development path.
Implement Strategic Risk Analysis: Identify potential risks early to prevent costly issues later.
Engage with Stakeholders: Get feedback from potential users, healthcare professionals, and regulatory experts to guide development.
2. Formulation, Concept, Feasibility, and Related Medical Device Development Costs
This phase involves translating the initial research into a tangible device concept and assessing its feasibility. A well-thought-out device design, considering all regulation requirements from the outset, can prevent costly redesigns later.
Early-stage feasibility studies and prototype testing help refine the concept, potentially reducing development time and costs.
Here are some specifics to keep in mind:
Early Prototype Testing: Develop and test prototypes to validate the concept and design. Now is the time to hash out design alternatives. Minimizing design changes later in the development process is an important goal. Why? It will likely be much more expensive to implement design changes in later phases.
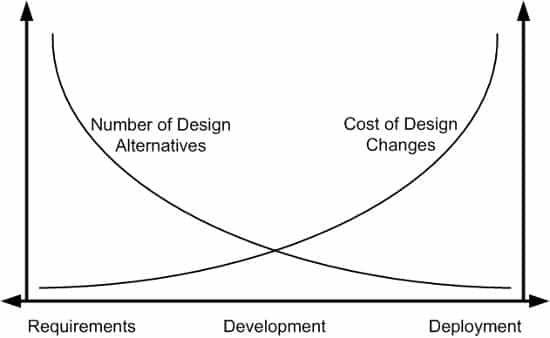
Regulatory Assessment: Review FDA requirements upfront to determine compliance requirements. If your plans include selling to international markets, be sure to check on regional regulatory requirements as well.
The FDA classification will significantly influence the device’s development costs and timeline in the US and abroad. Below is a general overview of how the FDA approaches device classification
Feasibility Study: Conduct a comprehensive feasibility study to assess the practicality and viability of the device.
3. Design and Development – Verification and Validation
The design and device product development phase is where the medical device takes shape. Adhering to safety standards and regulations is crucial during this stage. Developers must balance innovation with compliance to ensure device approval.
Implementing rigorous verification and validation processes early can identify issues sooner, avoiding delays and reducing costs associated with late-stage modifications.
Considerations for this phase include:
Iterative Design Process: Employ an iterative approach to refine the device based on continuous testing and feedback. For example, this is one area where SaMD developers benefit by using agile development methodologies to rapidly iterate software based on user feedback and testing results.
Robust Validation and Verification: Implement stringent testing processes to catch and address issues early.
Compliance Checkpoints: Regularly review and align with regulatory requirements to avoid non-compliance issues.
4. Final Validation and Product Launch Preparation
Final validation involves extensive preclinical testing and evaluation to ensure the device meets all necessary standards before launch. Medical device product launch planning includes finalizing marketing strategies and distribution plans.
Efficient planning and thorough testing during this phase can minimize the risk of post-launch issues, which can be costly and time-consuming.
Factors to keep in mind for this phase include:
Final Validation: Conduct extensive validation testing to ensure safety and effectiveness in the real world.
Final Regulatory Review: Ensure all regulatory documents and submissions are complete and accurate.
Launch Planning: Develop a detailed market entry strategy, including marketing, distribution, and post-market surveillance plans.
Moving Ahead with Managing Medical Device Development Costs
The bottom line for successful medical device development is that success requires much more than just technical expertise. Development teams with deep niche technical expertise who are new to the advanced medical device development process may not realize how complex and detailed the journey to product launch and market success is.
Experienced teams know that successfully navigating this landscape requires meticulous planning, strategic foresight, and the right partnerships.
At Galen Data, we have years of experience partnering with teams to help them manage their regulatory compliance journey. We keep tabs on the regulatory changes and innovations like AI and SaMD requirements. If you have questions about how to plan for the complexity of medical device development costs, reach out today to get the conversation started.