New FDA Regulations on Artificial Intelligence and Machine Learning in Medical Devices
The integration of Artificial Intelligence (AI) and Machine Learning (ML) in advanced medical devices is revolutionizing health care with innovative solutions from diagnostic support to personalized patient care.
As of October 2023, the FDA has approved 692 AI-enabled advanced medical devices. In 2023 alone, the FDA approved 108 devices.
However, AI innovation is just beginning. As such, it’s a moving target for regulators. The FDA is actively responding to this need, formulating regulations to ensure these advanced technologies are safe, effective, and reliable. This post will look at a summary of updated FDA actions, as well as some background information for the topic. Let’s dive in.
FDA Regulatory Updates for Advanced Medical Devices
The FDA’s AI/ML Software as a Medical Device Action Plan is a plan to ensure the safe and effective use of AI/ML in healthcare. The plan provides regulatory guidance for developing, validating, and deploying AI/ML-enabled devices.
The FDA is clearing an increasing number of AI/ML-based medical devices through the 510(k) pathway. This pathway allows clearance if the device is substantially equivalent to a former cleared device.
The FDA’s new guidance for AI in medical devices aims to provide flexibility for devices with continuously learning algorithms. The guidance also retains certain limits on the software to safeguard the devices’ continued safety and effectiveness.
The FDA also expects SaMD manufacturers to commit to transparency and real-world performance monitoring for AI/ML-based software as a medical device. The FDA also expects periodic updates on what changes were implemented.
Backing up a bit, advanced medical device teams may need to clarify the difference between AI and ML for stakeholders or investors new to the industry. Let’s look at a basic overview you can use.
What Is the Difference Between Artificial Intelligence and Machine Learning for Medical Devices?
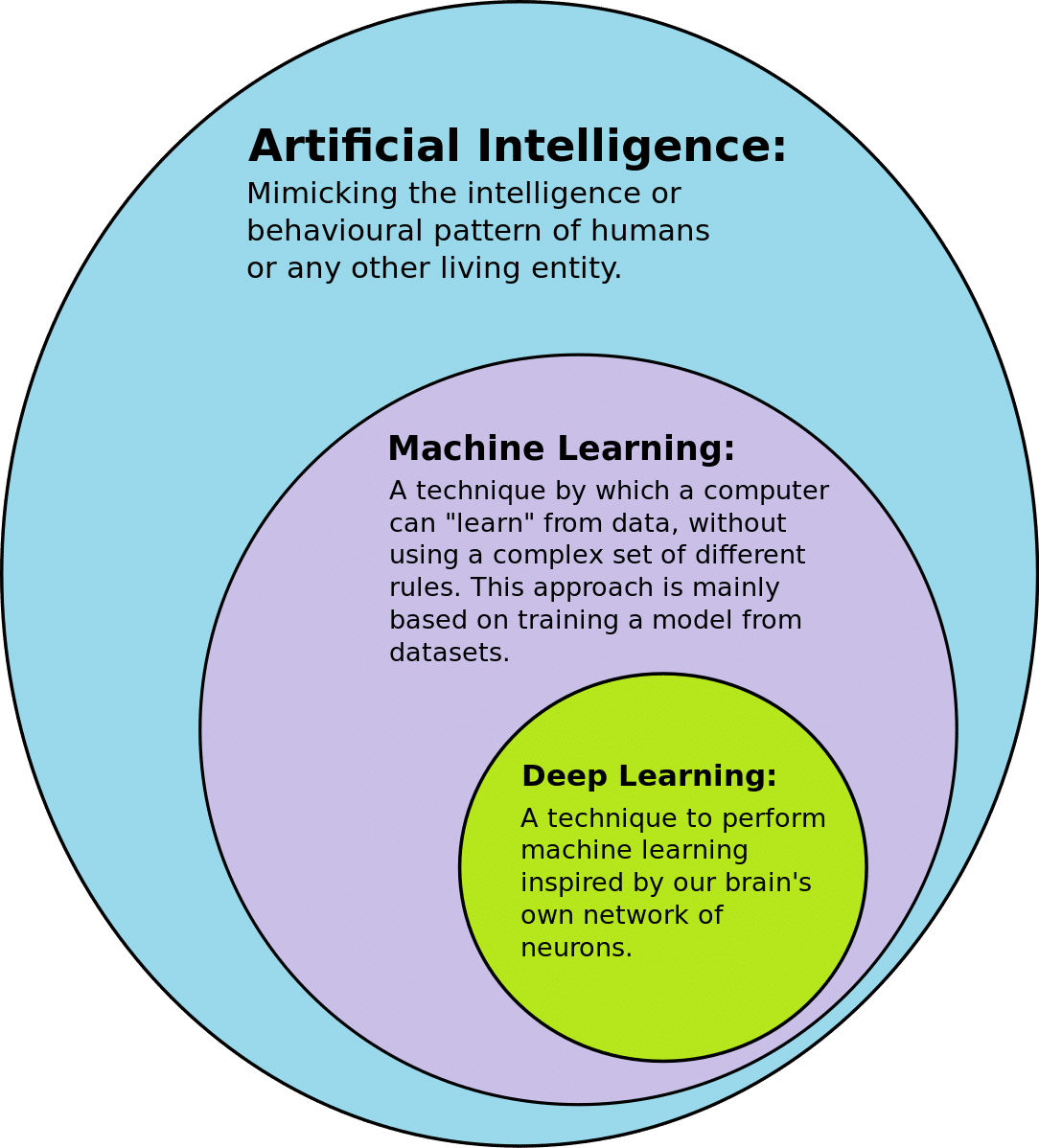
In the context of advanced medical devices, artificial intelligence and machine learning are closely related concepts with distinct differences. This graphic simplifies the differences:
Artificial Intelligence in Medical Devices
AI in advanced medical devices refers to the simulation of human intelligence in machines programmed to think and learn like humans, such as interpreting data, identifying patterns, and making decisions.
Application in Medical Devices: AI might be used to analyze complex medical data or aid in diagnostic processes. For example, AI-driven diagnostic tools, smart monitoring devices that analyze patient data for health trends, or robotic systems that assist in surgeries.
Machine Learning (ML) in Medical Devices
ML is a subset of AI focusing specifically on developing algorithms to learn from and make predictions or decisions based on data. The key characteristic is that ML algorithms learn and improve from experience instead of programmers writing code for every contingency. ML also incorporates deep learning systems that mimic how the human brain functions in learning.
Application in Medical Devices: ML is often used for pattern recognition and predictive analysis, such as an ML algorithm in a diagnostic device that learns to identify cancerous cells from medical imaging data. Over time, its accuracy and efficiency can improve with more exposure to data.
For example, ML algorithms used in radiology to detect abnormalities in imaging scans, wearable devices that learn to detect irregularities in physiological data, or predictive analytics tools for patient risk assessment.
The Growing Role of AI and ML in advanced medical devices
In 2015, the FDA approved 5 advanced medical devices with AI. Over the next six years, FDA approvals spiked exponentially, reaching 115 approvals in 2021. Even though the global pandemic disrupted that trajectory, the FDA authorized 155 additional devices since August 2022.
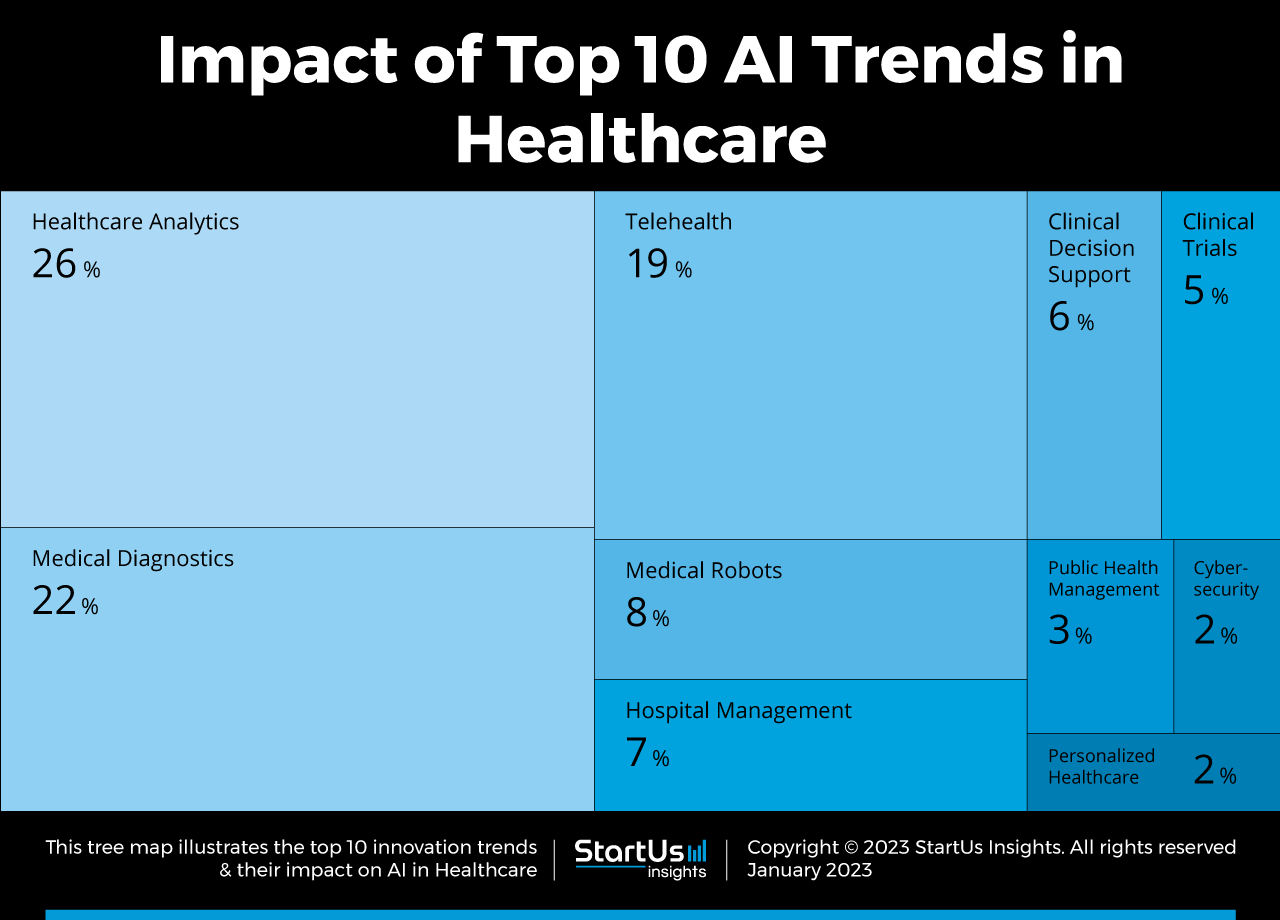
Although AI is poised to affect every aspect of digital health, it is influencing some sectors more than others. This 2023 chart from StartUs Insights shows the top 10 AI trends and AI’s current impact on advanced medical devices.
For example, by analyzing large data sets in life sciences, these technologies provide health care insights that human analysis alone cannot achieve. In diagnostics, artificial intelligence algorithms can detect patterns indicative of diseases, sometimes even before symptoms appear. In treatment, AI-driven devices tailor therapies to individual patients, enhancing treatment efficacy. This evolution promises to improve patient outcomes, streamline healthcare processes, and open new avenues for medical research and innovation.
The FDA Perspective on AI/ML in Medical Devices
Over the past few years, the FDA has been developing the regulatory framework for AI/ML in medical devices.
In 2022, the agency released a draft guidance proposing a science-based approach to requirements for advanced medical devices powered by AI and machine learning (ML).
One main takeaway is that the device’s algorithm will influence the relative ease of FDA approval. A locked algorithm results in more predictable behavior, allowing for more straightforward testing and validation processes.
In contrast, adaptive algorithms can modify their behavior based on real-time data and user interactions. This adaptability introduces additional complexities and challenges for FDA clearance or approval. Obtaining FDA clearance or approval with a locked algorithm is generally more straightforward than with an adaptive algorithm.
In a November 2023 update, the agency published an article on their Artificial Intelligence Program researching AI/ML-based medical devices. The purpose was to highlight the regulatory science research approach to ensure the safety and effectiveness of AI-enabled medical devices.
The article outlines the FDA’s action plan to address the unique regulatory challenges posed by integrating AI and ML in medical devices, particularly in ensuring patient safety, accuracy, and effectiveness.
It also details the specific challenges and gaps in regulatory science that the program aims to address, along with the activities and research areas the program focuses on to overcome these challenges.
Regulatory science gaps and challenges for AI/ML in medical devices include:
- Enhancing AI algorithm training for limited labeled data.
- Analyzing and minimizing bias in AI-enabled devices.
- Developing metrics for performance estimation and reference standards.
- Evaluating the safety and effectiveness of continuously learning AI algorithms and emerging clinical applications.
- Establishing methods for post-market monitoring of AI devices.
What is the focus of the FDA’s AI Program for AI/ML devices?
The FDA’s research activities for AI/ML focus on several areas. Knowing where their focus is can help advanced medical device stakeholders in their planning process:
- Data augmentation and synthetic data to improve algorithm training and testing.
- Methods to quantify and reduce algorithmic bias and ensure generalizability.
- Study designs and methods for AI/ML-based computer-aided triage.
- Developing new frameworks for continual learning and multi-class systems.
- Training and metrics assessment for machine learning algorithms.
- Evaluating the reliability of adaptive AI/ML algorithms and estimating the robustness of AI/ML against variations in data acquisition.
Moving ahead with FDA’s AI/ML Regulation
The FDA is proactively moving ahead to keep pace with innovation and demand for devices that incorporate AI/ML. While this may seem overwhelming, the advanced medical device industry is no stranger to cutting-edge innovation.
At Galen Data, we were among the first to optimize client processes for cloud-based reporting with online databases and Software as a Medical Device clients.
If you have questions about how AI will influence your regulatory journey, reach out today.