Speed to Market and Compliance in Medical Device Development
Advanced medical device development is an expensive and time-consuming process. Teams constantly juggle the tension between investor pressure for a market launch ASAP vs. running the regulatory gauntlet.
The North Star for compliance in medical device development is always going to be proving patient safety via regulatory compliance. Share on XWhen companies are in the choppy waters of development, figuring out how to get FDA approval for medical devices can feel like treading water. It’s helpful to remember that the North Star for compliance in medical device development is always going to be proving patient safety via regulatory compliance.
So, how do experienced teams navigate the balance between rapid development and market entry with unwavering adherence to stringent regulations? At Galen Data, we’ve helped many different types of Advanced medical device startups over the years. We’ll share some things we’ve learned along the way.
What Impacts Speed to Market vs. Compliance in Medical Device Development?
Advanced medical devices directly impact human health and well-being to varying degrees. In general, the more invasive the technology, the more stringent the regulations.
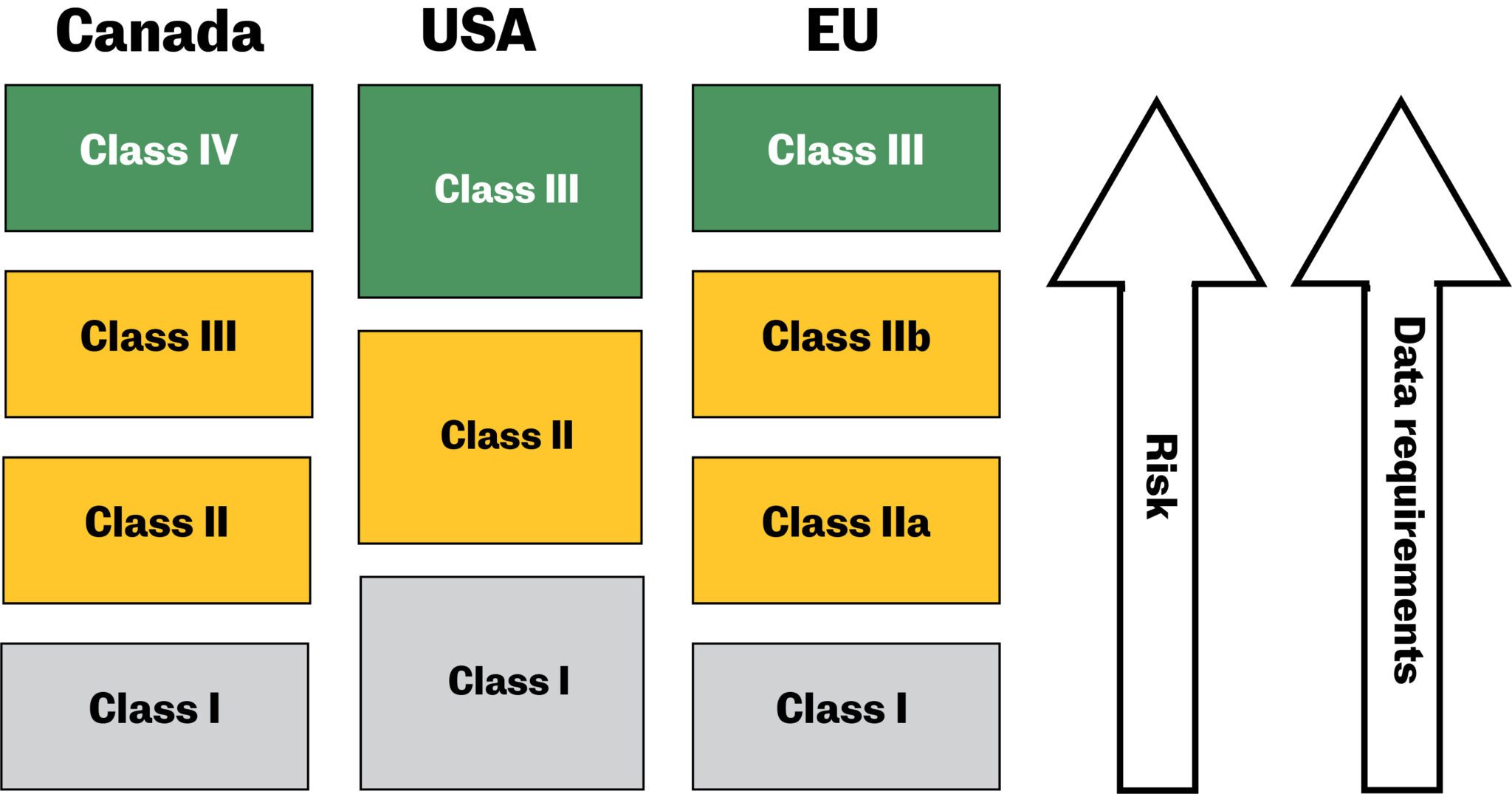
To address the fact that not all Advanced medical devices have the same risk profiles for patients, each agency monitors the development of medical devices to provide clearance by grouping devices into classes. Medical device compliance standards vary across the globe.
In the US, the FDA medical device classifications fall into three buckets. You can download the FDA guidelines for medical device pdf here. Developers interested in the Canadian market can find info and medical device regulations pdf at this website.
The EU medical device (EU MDR) classifications differ from the US and Canada.
For teams developing Advanced medical devices for the international market, understanding the differences between regional classifications early on is critical.
Balancing Priorities Between Compliance and Speed to Market
In Advanced medical device development, balancing competing priorities is no easy feat. Companies developing Advanced medical devices face a multitude of challenges:
- Ever-evolving regulations: Regulatory requirements differ across countries and are constantly evolving, making it challenging to keep pace.
- Data complexity: Generating and managing the vast amount of data required for regulatory submissions is a complex and time-consuming process.
- Resource constraints: Small and medium-sized companies often lack the resources and expertise to navigate regulatory hurdles.
No Shortcuts
As much as startups and investors might wish it were so, there are no shortcuts in medical device compliance. Failing to comply with regulations can lead to even more delays. Here are some risks of non-compliance:
- Patient harm: Defective or non-compliant devices can cause injury, illness, or even death.
- Regulatory penalties: Non-compliance can result in hefty fines, product recalls, and even market bans.
- Reputational damage: A lack of compliance can erode trust and damage a company’s reputation, making it difficult to attract investors and customers.
Key Factors Accelerating Advanced Medical Device’s Speed to Market
Companies are not powerless over the time it takes to get to market. While they can’t shortchange the regulatory process, they can try to remove friction in other areas. Here are some key factors that impact speed to market:
Design Innovation: Innovation is essential for growth in the medical device industry. It also improves patient outcomes over the long haul. However, as any seasoned device developer can tell you, innovation usually comes at a price.
FDA medical device regulations for an innovative device may include more regulatory scrutiny during clinical evaluation than a device with known technology or based on standard medical equipment, for example.
Production Efficiency: A good manufacturing phase may make up for lost time in the pre-approval phase. Efficient lean manufacturing principles, automation, and robotics can streamline production processes.
Exploring advanced manufacturing techniques like 3D printing can lead to faster iteration and customization, further accelerating your time to market.
Project Management Prowess: A well-oiled project management system is the backbone of a successful process. Medical device companies’ reporting requirements span the product development lifecycle, from concept to active marketplace presence. Data-driven decision-making, fueled by real-time insights, ensures transparency and keeps your team moving in the right direction.
Regulatory Navigation: Remember, speed cannot come at the expense of compliance. Partnering with regulatory experts and leveraging cloud-based data management solutions can streamline regulatory submissions and ensure you meet all requirements.
These solutions can automate data collection, organization, and analysis, saving you time and resources while ensuring your smooth and efficient compliance journey. At Galen Data, we have years of cloud solution expertise, we’re happy to chat if you have any questions.
AI’s Role for Compliance in Medical Device Development
Advanced technologies like artificial intelligence (AI) and machine learning (ML) are revolutionizing Advanced medical devices. One main takeaway is that the device’s algorithm will influence the relative ease of FDA approval.
A locked algorithm produces more predictable behavior, allowing for more straightforward testing and validation processes.
In contrast, adaptive algorithms can modify their behavior based on real-time data and user interactions. This adaptability introduces additional complexities and challenges for FDA clearance or approval. Obtaining FDA clearance or approval with a locked algorithm is generally more straightforward than with an adaptive algorithm. You can learn more about the latest FDA stance on AI for medical device manufacturers in this article.
Common Roadblocks in Medical Device Go-to-Market Timelines
While the allure of a speedy market launch is undeniable, the regulatory path presents unavoidable friction in the journey.
- Regulatory Roadblocks: You must navigate various regulatory hurdles depending on your target market. In the United States, obtaining FDA approval is the primary requirement. In Europe, the CE marking signifies compliance with the Medical Devices Regulation (MDR), and the specific requirements vary depending on the device’s classification.
- Clinical trials: Providing scientific evidence of your device’s safety and effectiveness is the cornerstone of the regulatory journey. Rigorous protocols ensure data integrity and ethical conduct, protecting participants while yielding valuable insights.
- Quality assurance: QA is an ongoing process that ensures your device meets all predefined quality standards throughout the entire development and manufacturing lifecycle. Medical device reporting with a robust QA system demonstrates your commitment to patient safety and transparency.
- Risk Management: Identifying and mitigating potential risks is crucial for compliance in the medical device development FDA process. Create a comprehensive plan to proactively address potential hazards, ensure product safety, and minimize the possibility of adverse events.
- The Power of Partnership: Partnering with regulatory experts can be invaluable. They possess in-depth knowledge of the complex regulatory landscape and can guide you through the submission process, ensuring your application meets all requirements.
Minimize data and reporting friction risk from the start. Utilize a cloud-based data management solution that can streamline data collection, organization, and analysis, saving time and resources while ensuring your compliance journey is efficient and transparent.
Speed with Safety: Accelerate Market Entry While Maintaining Compliance
The good news is that achieving faster market entry without compromising compliance is possible. Here are some key strategies:
- Embrace Regulatory Expertise: Partnering with regulatory consultants can help you navigate the complex regulatory landscape efficiently. They can guide you through the submission process, ensure your application meets all requirements, and anticipate potential roadblocks.
- Leverage Technology: Cloud-based data management solutions can streamline data collection, organization, and analysis for regulatory submissions. This saves time and ensures your compliance journey is efficient and transparent.
- Adopt Lean Manufacturing: Implementing lean manufacturing practices optimizes production processes, minimizes waste, and reduces turnaround times. Automation and robotics can further accelerate production and ensure consistent quality.
- Foster Partnerships: Partnering with experts in various fields, such as clinical trial research organizations (CROs) or contract manufacturers (CMOs), can provide valuable resources and expertise, freeing your team to focus on core development activities.
- Prioritize Quality: While aiming for speed, never compromise on quality. Implementing a robust quality management system (QMS) ensures consistent product quality, reducing the risk of regulatory delays or recalls.
- Embrace Continuous Improvement: Foster a culture of continuous improvement within your organization. Regularly assess your processes, identify areas for improvement, and implement changes to streamline your go-to-market process while maintaining compliance.
By implementing these strategies, you can achieve a faster, smoother journey to market while ensuring the safety and efficacy of your medical device – ultimately benefiting patients and your company’s success.
Moving Ahead with Compliance in Medical Device Development
Balancing speed to market with regulatory compliance, manufacturing and other issues is a major key to success. Data management and security is one of the most important factors to get right. Be sure to consider cloud connectivity early in the design roadmap to mitigate risk and take advantage of potential benefits.
Contact us today if you’re unsure how to manage cloud connectivity considerations for your product. We can help you assess the pros and cons and make an informed decision for your product development roadmap.