How to Find Law Firms for the Advanced Medical Device Regulatory Journey
The regulatory landscape for advanced medical devices is among the most challenging in health care. From ensuring compliance with FDA regulations to protecting intellectual property, having the proper legal guidance is crucial for the success of any medical device company.
Finding the right law firm for the advanced medical device regulatory journey requires a focused approach, ensuring that the firm you choose has the expertise and connections necessary to help you thrive in this highly regulated industry.
But first of all, you may be wondering, why you need legal support in the first place. Isn’t navigating the regulatory gauntlet part of the Product Development Partner’s purview?
Let’s take a look.
Why Medical Device Companies Need Specialized Legal Support
You already know that medical device companies operate in a complex and heavily regulated environment. The graphic below provides an overview. Where there is regulation, there are lawyers because, at every point along the way, the potential exists for legal questions or clarification.
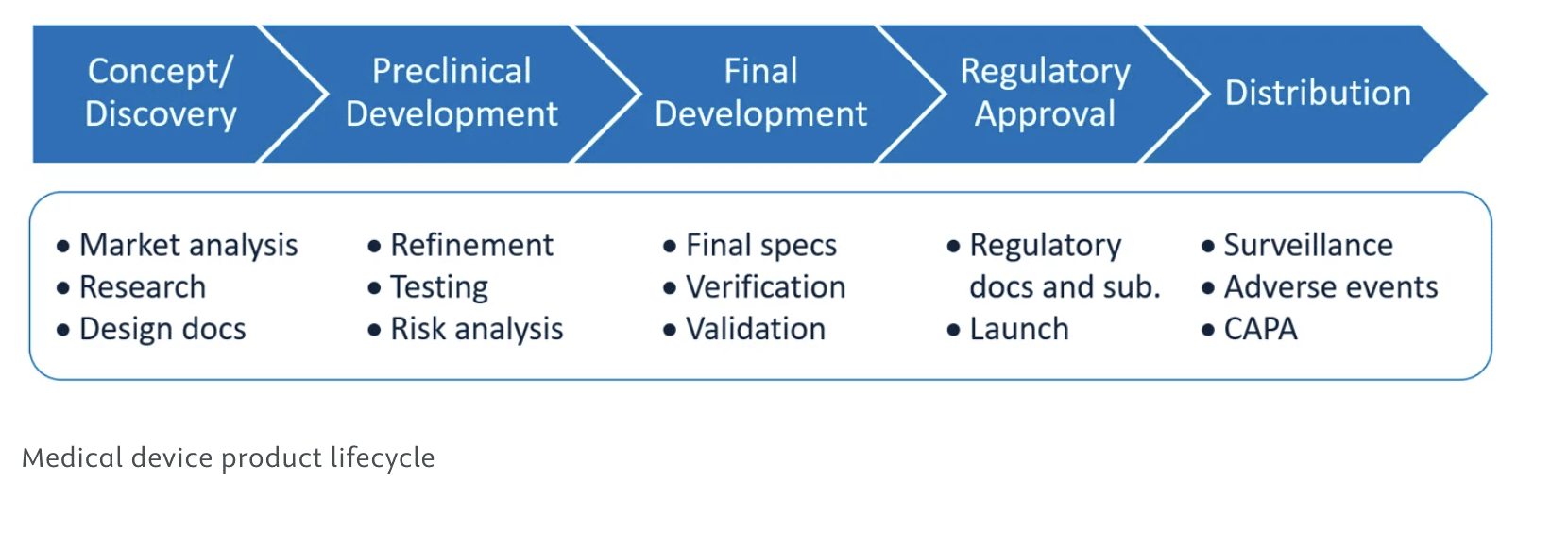
The regulatory requirements imposed by the FDA and other global regulatory bodies demand that companies adhere to strict guidelines. Specialized legal support comes into play for several key reasons:
Regulatory Compliance and Quality Management
Compliance with FDA regulations is mandatory for developing, testing, and marketing medical devices in the United States. Law firms with expertise in regulatory compliance help ensure that your device meets all necessary standards before it hits the market.
Beyond regulatory affairs, maintaining robust quality management systems is critical to the success and safety of medical devices. A firm well-versed in the medical device industry will guide you in establishing and maintaining these quality systems, mitigating risks associated with non-compliance.
Intellectual Property Protection
Patents are vital in the industry. Given the rapid pace of innovation in life sciences and digital health, protecting your intellectual property is essential to maintain a competitive edge.
A specialized firm can assist in securing and defending your patents, preventing competitors from infringing on your intellectual property. They can also help devise a robust regulatory strategy that aligns with your innovation goals.
Product Liability and Risk Management
If a medical device fails, the consequences can be severe, both legally and financially. Firms specializing in product liability and managing risk can help you mitigate these risks and defend your company in litigation.
They provide crucial support in understanding how similar court rulings can significantly impact your defense strategy.
Corporate Transactions and Regulatory Affairs
Mergers, acquisitions, and partnerships are common in the industry. Legal expertise is crucial to structuring these deals to maximize value and minimize risk. Additionally, as the industry increasingly intersects with digital health, having legal counsel who understands both traditional medical devices and emerging healthcare technologies is invaluable.
Clinical Trials and Strategy for Your Regulatory Journey
Device trials are an essential part of bringing new medical devices to market. However, they come with their own set of regulatory challenges. Firms with a deep understanding of the clinical phase can help you navigate these complexities, ensuring that your trials comply with FDA regulations and global standards.
This is a critical component of a broader strategy that considers every aspect of your device’s journey to market.
How to Find Law Firms for the Advanced Medical Device Regulatory Journey
Finding the right firm involves more than just a quick online search. It requires careful research and consideration of the firm’s expertise, reputation, and connections within the industry. Here’s how to approach this process:
Research Industry-Specific Rankings
Start by exploring legal directories that rank firms based on their expertise in life sciences, healthcare, and FDA law:
Chambers and Partners: This directory ranks firms and attorneys based on client feedback and legal expertise. Look for firms highly ranked in life sciences, healthcare, and the medical device space.
The Legal 500: Similar to Chambers, The Legal 500 provides detailed rankings and reviews of firms across various industries, including medical devices, regulatory issues, and quality oversight.
Industry Publications
Articles and reports in industry publications may highlight notable cases or client experiences, offering additional insights into a firm’s expertise in the industry.
LMG Life Sciences: This publication highlights leading firms and attorneys in the industry. Firms recognized here are often leaders in regulatory issues, intellectual property, and risk mitigation.
MedTech Insight: This resource often recognizes firms that excel in the medical device and health tech sectors, providing crucial insights into regulatory issues and quality systems.
Law Firm Websites
Many firms showcase their successful representations in FDA regulatory matters, patent litigation, the clinical trial phase, and corporate transactions on their websites. Case studies can illustrate how a firm has effectively managed complex regulatory strategies for other medical device companies.
Industry Peers, Networks, and Associations
Engaging with industry peers and professional networks to gather recommendations from associations can be a great way to get word-of-mouth recommendations.
AdvaMed (Advanced Medical Technology Association): As a leading association in the medical technology sector, AdvaMed can be a valuable resource for identifying top firms specializing in FDA compliance and quality oversight.
MassMEDIC (Massachusetts Medical Device Industry Council): This council also provides insights and recommendations on leading legal experts in the field, particularly those with a strong focus on the intersection of devices and digital innovation.
Evaluate Firm Expertise
When evaluating firms, prioritize those with the specialties you need. For example, dedicated life sciences or health care practice groups may have attorneys with a background in biomedical engineering or FDA regulatory experience often bring specialized knowledge that’s particularly beneficial for medical device firms.
This expertise can be crucial in developing a strategy that aligns with your innovation goals and stringent industry requirements.
Firms that employ attorneys who have served as in-house counsel or within the broader healthcare industry can offer unique perspectives and practical insights, particularly in managing risk and regulatory issues.
Beyond Expertise: The Power of a Law Firm’s Network
When selecting a law firm for your advanced medical device regulatory journey, expertise is undoubtedly crucial, but it shouldn’t be the sole factor in your decision-making process. A law firm’s network, particularly its connections and pre-existing relationships with key regulatory bodies like the FDA, can be invaluable.
Firms with strong relationships at the FDA often have a deeper understanding of the agency’s expectations and can provide insights beyond written regulations.
Moreover, a firm’s network that extends beyond the FDA to include connections within the broader medical device and life sciences communities can help you access a wealth of resources, insights, and opportunities that can significantly enhance your company’s ability to succeed.
Examples of Top Law Firms for the Advanced Medical Device Regulatory Journey
Below are a few firms frequently recognized for their work in the medical device and healthcare digital innovation sectors:
Goodwin Procter: Goodwin Procter sits at the intersection of medical technology and the law, serving as a single-point solution for medtech clients seeking to maneuver through this evolving and complicated web of laws, regulations, guidance, and industry standards.
Wilson Sonsini: Known for representing medical technology companies at all stages, from formation to exit. A full-service firm, Wilson Sonsini also specializes in areas vital to early-stage enterprises, particularly intellectual property and finance.
Covington & Burling LLP: Known for its FDA regulatory practice and strong team, Covington & Burling is a top choice for navigating complex regulatory environments and ensuring compliance across global markets.
WilmerHale: With a focus on intellectual property, regulatory issues, and risk mitigation for devices, WilmerHale offers comprehensive legal support for companies looking to protect their innovations and comply with regulatory requirements.
Hogan Lovells: This global firm has a renowned practice with extensive FDA expertise, making it a strong partner for companies navigating the complexities of compliance and quality oversight.
Next Steps in the Regulatory Journey
Choosing the right firm is a critical step in your regulatory journey. Whether you’re focused on FDA compliance, quality oversight, or risk mitigation, look for firms with specialized knowledge to support your company at every stage of development.
At Galen Data, we understand the unique challenges of medical device data and have the cloud expertise to help you navigate them. Partner with Galen Data today to develop a secure and scalable data management plan, leverage our data and compliance expertise, and focus on innovation while we handle the infrastructure.
Schedule a call with us today to discuss your specific needs and see how Galen Data can help you store, manage, and secure your data at scale.