AI-Driven Risk Management in Medical Device Development
Digital health and AI technology are rapidly transforming healthcare and medical device development. What started out as a process for creating tangible devices and medical products now increasingly includes a device software component.
With the increasing complexity of medical devices, companies are turning to AI-driven solutions to help them manage risks more effectively.
Artificial intelligence in risk management is rapidly becoming a health care game-changer, allowing manufacturers to assess risks earlier, streamline processes, and ensure devices meet stringent global regulatory standards.
In a general sense, AI helps improve and automate risk management across 3 phases: Identifying, analyzing, and managing.
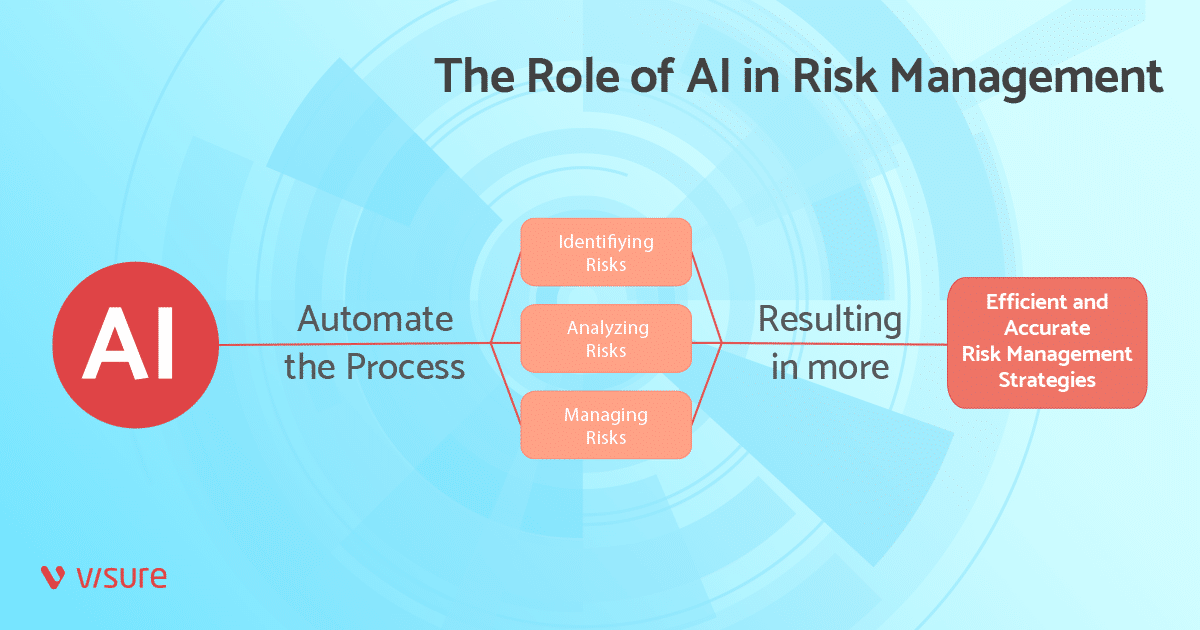
In this article, we’ll explore how artificial intelligence and machine learning are reshaping risk and compliance strategies across the medical device journey.
Identifying and Assessing Risks with Artificial Intelligence
Traditional risk identification is a manual, time-consuming process. Engineers and quality assurance teams must analyze large datasets, review historical performance data, and assess every potential point of failure. Even with diligent effort, human error or oversight can still occur.
Good machine learning practices are revolutionizing risk assessment. By adhering to robust data handling, ethical AI guidelines, and model transparency, machine learning algorithms allow medical device companies to analyze vast amounts of data in real-time with high accuracy.
Predictive AI-powered analytics can sift through historical data, clinical trial results, and real-time device performance to flag areas vulnerable to failure.
These AI systems don’t just detect risks—they also prioritize them. By evaluating the severity and likelihood of each risk, machine learning helps medical device companies allocate resources efficiently, focusing on the most critical issues first.
One example is in post-market surveillance. After a medical device launch, AI systems can proactively monitor real-world performance, catching potential issues faster than traditional methods. This approach reduces the risk of device failure and enhances patient safety.
Mitigating Risks Through Artificial Intelligence
Identifying potential risks is the first step. The next is to mitigate those risks effectively, particularly for devices that directly impact patient care, such as those integrated with clinical decision support systems.
Traditional risk mitigation involves manual processes like human audits, design reviews, and lengthy testing procedures. While these steps are essential, they can be resource-intensive and may not always catch hidden vulnerabilities.
This is where AI-driven risk mitigation comes into play. AI’s ability to process large datasets and run real-time simulations offers medical device companies a significant advantage in mitigating risks. For example, models can simulate various scenarios based on historical data and real-time device performance, allowing engineers to anticipate weaknesses before they become critical.
Another powerful feature of AI in risk mitigation is anomaly detection. AI systems are highly adept at recognizing deviations from expected behavior, even when those deviations are subtle.
For medical devices, this capability can alert companies to potential malfunctions or design flaws that may not be obvious through traditional testing. By catching anomalies early, AI reduces the likelihood of patient harm or costly product recalls.
In addition to improving device safety, AI can streamline the risk mitigation process. Tasks that once required human intervention, such as updating risk protocols or documenting design changes for compliance, can now be automated. This saves time and ensures that risk mitigation strategies are always up to date and aligned with the latest industry standards.
Regulatory Compliance and AI in Medical Devices
Everyone in the industry knows that regulatory sign-off is the most significant hurdle in developing medical devices. With the rise of AI in medical device design and development, new opportunities now exist to ensure compliance.
Regulatory compliance is a critical aspect of medical device development, requiring companies to provide extensive documentation to meet FDA and international standards. Traditionally, this involves using QMS software for medical devices to manage documentation, track corrective actions, and maintain a consistent quality process.
One of AI’s key advantages is automating the documentation creation and maintenance required for compliance. For instance, AI systems can track every step in the risk mitigation process, automatically logging data and generating reports that meet FDA and international standards like ISO 14971.
AI’s real-time monitoring capabilities also enable medical device companies to maintain compliance more efficiently. By continuously analyzing data, AI can identify emerging risks and alert companies to potential regulatory violations before they become serious issues.
This proactive approach reduces the chance of non-compliance, which can lead to costly delays in product approval or even regulatory penalties.
Moreover, regulatory bodies increasingly acknowledge AI’s role in risk management. The FDA, for example, has begun issuing guidelines on using AI and machine learning in medical devices, particularly in risk mitigation processes.
AI’s ability to enhance transparency and traceability makes it a powerful tool for demonstrating compliance. By using AI, companies can ensure that their medical devices’ risk management practices meet current regulatory standards and are future-proof as regulations evolve.
Future of AI in Risk Management
As artificial intelligence technologies evolve, their applications in medical devices will become even more sophisticated, leading to faster, safer, and more compliant development processes. One of the most promising developments on the horizon is the use of adaptive AI systems.
Adaptive AI systems have the potential to continuously learn and respond based on real-world data. For device companies, this means AI could predict and mitigate risks and evolve its risk strategies as new data becomes available. This will be especially valuable for devices operating in changing environments or those requiring post-market surveillance to ensure long-term safety.
Another growth area is the increasing integration of AI into regulatory frameworks. As regulatory bodies like the FDA and international agencies recognize the benefits of AI, we can expect to see more guidance and standards emerge specifically for AI-driven medical devices.
Furthermore, AI will likely play a more significant role in post-market activities, particularly in predictive maintenance. By analyzing real-time data from devices in the field, AI can forecast potential failures before they happen, allowing for proactive repairs or updates. This predictive capability will help improve patient outcomes and reduce the risks associated with device malfunctions.
Lastly, AI-driven digital twins—virtual replicas of physical devices—are set to revolutionize risk mitigation. By simulating real-world performance, digital twins can help companies test their devices under various conditions without risking actual patient safety. These simulations will enable developers to identify and address risks in a virtual environment, accelerating the design and testing phases while reducing costs.
Moving Ahead with AI-Driven Risk Management
AI is no longer just a tool of the future—it is transforming the medical device industry today. At Galen Data, we understand the unique challenges of developing safe and compliant devices.
Our secure and scalable cloud platform is built to help you leverage the power of AI for risk management and compliance, allowing you to focus on what matters most: innovation and patient safety.
At Galen Data, we have the cloud expertise and regulatory knowledge to support your company at every stage, from design to post-market surveillance. Reach out today to learn more about how you can leverage our expertise in medical device data and compliance.