Future Predictions and Advanced Medical Device Industry Market Dynamics in 2025
As we move toward 2025, the medical device industry continues its post-COVID growth trajectory. Rapid advancements in medical technology, evolving healthcare demands, and the push for personalized care drive innovation in the field. Advanced medical devices are at the heart of this evolution, reshaping patient outcomes, improving operational efficiencies, and opening new markets.
Understanding the advanced medical device market dynamics for 2025 is essential for founders and consultants to stay competitive. This post explores the industry’s key trends, challenges, and growth drivers, offering actionable insights to help medical device companies thrive in an increasingly complex ecosystem.
Advanced Medical Devices Market Dynamics and Growth Projections
Like the rest of health care, the advanced medical device market is entering a transformative phase, driven by technological innovations and global healthcare challenges. The demand for more innovative, efficient medical solutions is growing due to aging populations, the rise in chronic diseases, and the shift toward value-based care.
Market analysts predict a steady growth rate of 5.68% annually (CAGR 2024-2029), leading to a projected market volume of US$669.70bn by 2029, with cardiology devices leading as the largest category.
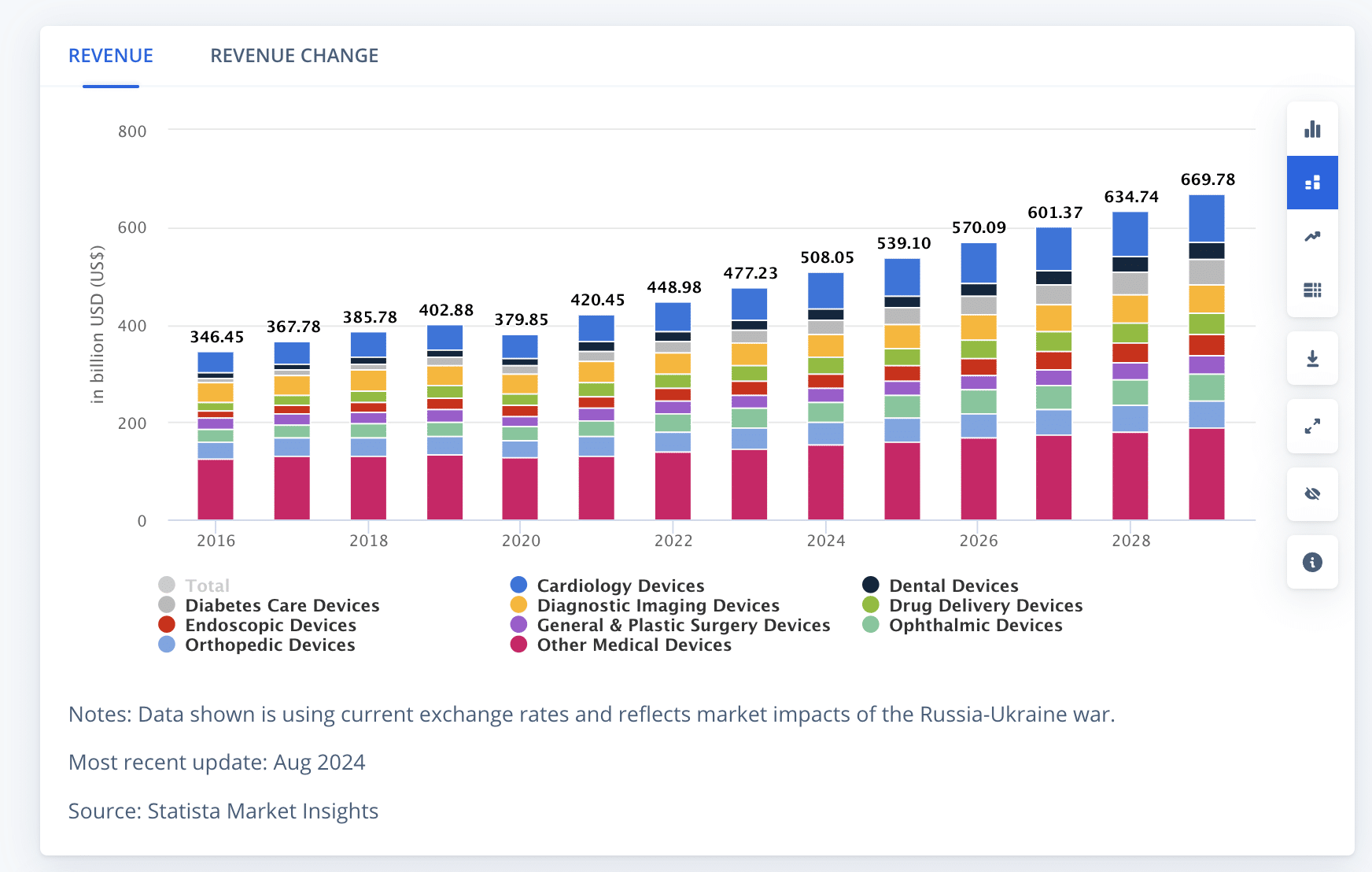
When considering the global landscape, the United States, with an aging population, high incidence of lifestyle diseases, and relative affluence represents the largest single-country market for industry growth.
The United States, with an aging population, high incidence of lifestyle diseases, and relative affluence represents the largest single-country market for industry growth. Share on XArtificial Intelligence: A Game-Changer in Medical Device Development
Artificial Intelligence (AI) is at the forefront of this revolution, enabling groundbreaking advancements in medical device development. AI-driven algorithms are augmenting advances in digital health, helping to design devices with enhanced precision, improve diagnostics, and streamline regulatory approval processes.
For example, machine learning models can predict patient outcomes with remarkable accuracy, enabling devices that monitor and anticipate health issues.
AI is also transforming clinical trials by analyzing vast amounts of patient data in real time, reducing development timelines, and cutting costs. Moreover, its integration into connected devices—such as wearables and remote monitoring systems—is redefining how patients and providers interact with technology.
Global Medical Devices Market Drivers and Challenges
While innovation fuels growth, medical device companies face several challenges. Regulatory compliance remains a critical hurdle, especially as global standards become more stringent. Additionally, the rising cost of research and development necessitates strategic investment in technologies that offer scalability and long-term value.
Despite these obstacles, emerging markets in regions such as Asia and Latin America are becoming key growth drivers, offering untapped opportunities for device companies. These markets foster innovation while increasing access to life-changing technologies, further accelerating the industry’s expansion.
By understanding these dynamics and leveraging cutting-edge technologies like AI, companies can navigate these challenges and position themselves as leaders in the future of healthcare. Platforms like Galen Cloud play a crucial role by offering secure, compliant, and scalable solutions tailored to the industry’s unique needs.
The Role of Healthcare Integration and Data Interoperability
Seamless data integration is revolutionizing the advanced medical devices industry. Medical devices no longer operate in isolation; they function as critical nodes within interconnected healthcare ecosystems. Interoperability enables these devices to share real-time data across platforms, improving patient care and operational efficiencies.
Healthcare providers need medical devices that integrate smoothly with electronic health records (EHRs), cloud platforms, and other digital systems. This demand drives device companies to prioritize interoperability during product development. The ability to securely collect, transmit, and analyze patient data without delays improves diagnostic accuracy and streamlines treatment workflows.
Regulatory agencies, including the FDA and global health organizations, are raising the bar for data-sharing standards. Non-compliance with these standards results in costly delays and limited market access. Companies that adopt robust, compliant solutions for data management gain a competitive edge.
Galen Data meets this need by providing a secure and scalable cloud platform tailored to medical devices. Its solutions help clients achieve full compliance with data regulations while enabling interoperability. This ensures faster time-to-market and greater confidence among stakeholders.
Interoperability and the Patient-Centered Future
Interoperability doesn’t just benefit providers; it transforms the patient experience. Patients using advanced devices, such as wearable monitors or implantable technologies, expect seamless integration with their care teams. Data interoperability enables this connection, ensuring providers receive accurate, real-time insights into patient health.
For example, a patient’s wearable device can alert a physician to abnormal vitals in real time, triggering immediate intervention. By leveraging cloud platforms, companies can design devices that provide these benefits while meeting the highest security and compliance standards.
Emerging Technologies Shaping the Advanced Medical Device Future Trends
The advanced medical devices market will witness transformative technologies in 2025, redefining the boundaries of innovation. Artificial Intelligence (AI), already a cornerstone, will integrate more deeply with robotics, enabling future medical devices with autonomous surgical systems and devices capable of predictive diagnostics. 3D printing will also revolutionize device prototyping and manufacturing, offering personalized solutions such as patient-specific implants and prosthetics at scale.
The Internet of Medical Things (IoMT) will expand further, connecting a network of smart medical devices that continuously monitor patient health. These systems will leverage real-time data analytics to enable remote patient management, reduce hospital readmissions, and enhance preventive care. Companies embracing IoMT technologies position themselves as leaders in the shift toward value-based healthcare.
Regulatory Evolution and Compliance Challenges
As technologies evolve, regulatory bodies will adapt their guidelines to address emerging risks and opportunities. The FDA and other global agencies are sharpening their focus on cybersecurity in medical devices, given the increasing connectivity of IoMT systems. Companies must proactively address these evolving standards to avoid delays and ensure market readiness.
Companies that adopt advanced cloud platforms, such as Galen Cloud, can stay ahead of these regulatory changes. By integrating security and compliance into their data management strategies, they ensure that their devices meet the rigorous demands of global markets.
Market Expansion into Emerging Regions
While North America and Europe dominate the advanced medical devices market, emerging economies in Asia, Latin America, and Africa will play a significant role in the industry’s future. These regions offer untapped growth potential, driven by expanding healthcare infrastructure and rising demand for innovative devices. Companies entering these markets must tailor their strategies to local needs, balancing cost-effectiveness with high-quality solutions.
Future-Proofing for Advanced Medical Device Companies
The advanced medical device industry is on the cusp of unprecedented change. Companies must embrace cutting-edge technologies, prioritize interoperability, and address ethical and sustainability challenges to thrive in this dynamic landscape. Platforms like Galen Cloud provide the tools and expertise to navigate these complexities, enabling companies to focus on innovation while ensuring compliance and security.
At Galen Data, we understand the unique challenges of the advanced medical device market. Our secure and scalable cloud solutions help companies manage data seamlessly, achieve regulatory compliance, and position themselves for success in 2025 and beyond.
Partner with Galen Data today to:
- Develop a secure and scalable data management plan.
- Leverage our expertise in medical device data and compliance.
- Focus on innovation while we handle the infrastructure.
Schedule a call with us today to discuss your specific needs and see how Galen Data can help you store, manage, and secure your medical device data at scale.