Reducing AI Risk to Secure Regulatory Approval
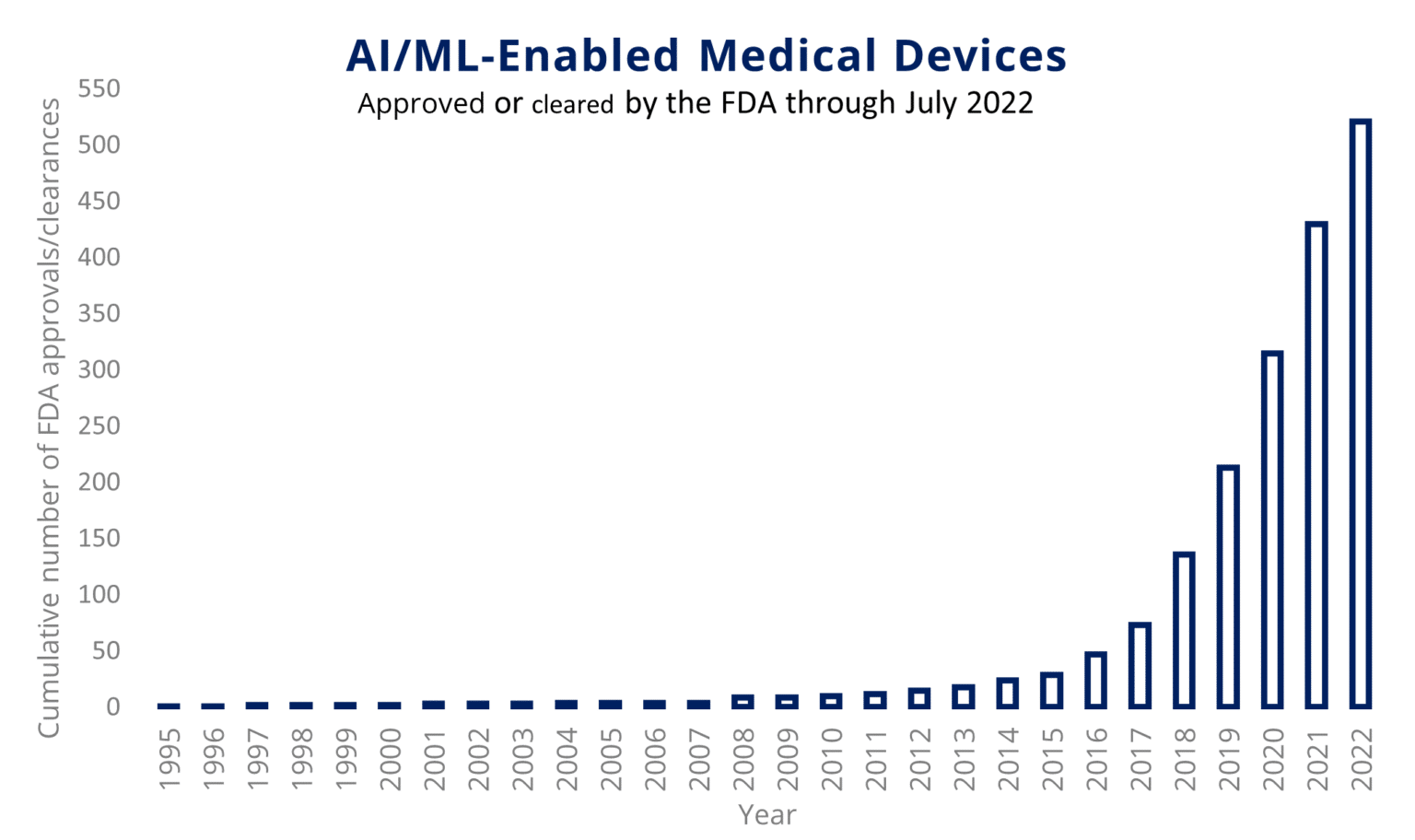
For most of 2023, the consumer tech space has been flooded with news about AI. As the chart below shows, the advanced medical device industry has been flirting with AI for several years.
What we are seeing now is AI+ advanced medical device are moving up the curve exponentially. Share on XThe implications for advanced medical device development are substantial, especially as regulatory bodies grapple with issues around data privacy and bias.
Whether you’re developing a tangible advanced medical device or a Software as Medical Device (SaMD) with AI, it’s essential to be aware of the potential risks associated with this technology. To increase understanding of this evolving topic, our Galen Data Medical Device Innovation webinar series recently featured Isabella Schmitt, MBA, RAC. Isabella is Director of Regulatory Affairs at Proxima Clinical Research, where she leads 100+ medical device and pharma projects and oversees the marketing team.
Stakeholder Importance in Advanced Medical Device Development
One of the most important tips is to engage early and often in the development process with various stakeholders, including physicians, end users, and regulators. Their input and feedback are invaluable in improving device functionality and safety.
Locked vs. Adaptive Algorithms for Advanced Medical Devices
Regulatory approval planning with AI focuses on algorithms and data risk. By addressing bias in data collection and curation, algorithmic improvements, and testing and evaluation, developers can ensure generalizability in AI algorithms to ensure they can be applied to diverse patient populations.
One key takeaway is that the type of AI algorithm affects the odds of FDA approval. A locked algorithm operates based on predefined rules and fixed parameters, making its behavior more predictable and allowing for more straightforward testing and validation processes.
In contrast, adaptive algorithms can modify their behavior based on real-time data and user interactions. This adaptability introduces additional complexities and challenges for FDA clearance or approval:
Obtaining FDA clearance or approval for a medical device with a locked algorithm is generally more straightforward compared to an adaptive algorithm for several reasons:
- Predictability: A locked algorithm operates based on predefined rules and fixed parameters, making its behavior more predictable. This allows for more straightforward testing and validation processes.
- Stability: A locked algorithm remains unchanged once deployed, ensuring consistent and reliable results. This stability simplifies the evaluation of safety and effectiveness over time.
- Control: With a locked algorithm, the manufacturer has complete control over the device’s behavior and can document its intended use, limitations, and potential risks.
- Validation: The validation process for a locked algorithm involves testing the device against a specific set of inputs and expected outputs. This can be more easily demonstrated and verified during the FDA review process.
For detailed information and specific guidelines, visit the FDA’s official website.
AI Data Risk for Advanced Medical Devices
“Garbage in, garbage out” has been a truism for data and tech development for decades. We could update that for the age of AI by saying “Bias in, bias out”.
Let’s look at two important areas relating to AI data integrity and risk for advanced medical device teams – data quality and bias, and transparency.
AI data quality and bias
AI data bias can stem from data imbalance, data quality problems, and algorithmic limitations. The problem for medical devices is that bias poses a significant risk to the quality of patient care. Imbalanced data can lead to skewed predictions and recommendations, potentially worsening health disparities.
Advanced medical device developer teams can employ different strategies to mitigate these risks and enhance data transparency. These approaches can help uncover and fix bias within the AI system:
- Meticulous data collection and curation, ensuring a representative and diverse dataset.
- Algorithmic improvements including techniques like hypothesis testing, visualization, and model interpretability.
- Robust testing and evaluation procedures, continuously assessing the AI’s performance for bias and fairness.
Assembling a diverse team is key to mitigating potential biases. Teams with varied backgrounds and perspectives can offer unique insights into bias detection and mitigation, helping to create a more balanced and fair AI system.
By addressing bias in AI data through these methods, medical device companies can enhance their algorithms’ accuracy and ensure equitable access to quality healthcare for all patients.
Data transparency
Enhancing data transparency in AI for advanced medical devices is crucial for building trust, ensuring accountability, and enabling continuous improvement.
Several challenges exist, including the difficulty in explaining algorithms and the need for more standardization in AI. Additionally, IP-related issues, such as privacy concerns and intellectual property protection, can hinder transparency efforts.
To overcome these challenges, advanced medical device developer teams can consider several strategies:
- Interpretable Models: Develop AI models that are interpretable and explainable. This means making the inner workings of the AI system understandable to humans, including healthcare professionals.
- Data Provenance and Documentation: Provide comprehensive documentation about the data sources, their quality, and any preprocessing steps. This helps users understand the data’s origins and potential biases.
- Clear Communication and Decision-Making: Maintain clear and transparent communication throughout the AI development process—document decision-making processes, including why specific algorithms or data sources were chosen, to promote accountability.
- Visualization and User Interfaces: Use visualization techniques and user-friendly interfaces to present AI-generated insights and predictions. This makes it easier for medical professionals to comprehend and trust the AI’s outputs.
- Transparency and Reproducibility: Establish transparent and reproducible research practices. Clear communication with stakeholders helps build trust and accountability in AI device development.
By implementing these strategies, developers can work toward greater transparency in AI for advanced medical devices. Fostering trust and ensuring that AI technologies are used effectively and responsibly will be helpful in the FDA approval process.
Key Points for AI Monitoring for Advanced Medical Devices
Monitoring is not only a necessity but also an ongoing opportunity for improvement. Here are some key ideas:
- Post-Market Monitoring: Continuously monitor AI devices after they enter the market to ensure ongoing safety, effectiveness, and improvement. This step is crucial for addressing emerging issues and enhancing device performance.
- Performance Evaluation: Employ model performance techniques, such as supervised monitoring, to evaluate the AI device’s performance over time. This allows for the identification of potential issues and the implementation of necessary improvements.
- Data Analysis Techniques: Utilize data analysis methods such as clustering and principal component analysis (PCA) to identify patterns and trends in the data generated by the medical device. These techniques help detect anomalies, improve device performance, and ensure data quality.
- User Behavior Monitoring: Continuously monitor user behavior and interactions with the medical device. This data can provide insights into the device’s effectiveness and help identify any issues that need attention. Understanding how users interact with the device can lead to user-centric improvements.
By implementing these techniques, medical device developers can actively monitor and enhance their devices in real-world scenarios, ensuring they meet the highest performance, safety, and user satisfaction standards.
Moving Ahead
In the complex landscape of medical device innovation, AI integration holds immense promise and presents new challenges on the regulatory front. At Galen Data, we are technologists with a deep understanding of the advanced medical device process. We have been at the forefront of adjusting quickly to tech innovations for several years.
Whether you are just starting planning or in the midst of an active project, we can help. Reach out for a call or a demo today.