Riding the Innovation Wave: 5 Top Medical Device Companies Known for Innovation
Like the Apollo moon shot in the 1960s to today’s Mars Rover explorations showcase exponential leaps in technology, the medical device field has also evolved at a mind-boggling pace. As new technologies like AI and cloud drive innovation, where should advanced medical device leaders look for direction and inspiration while channeling the vision into the here and now?
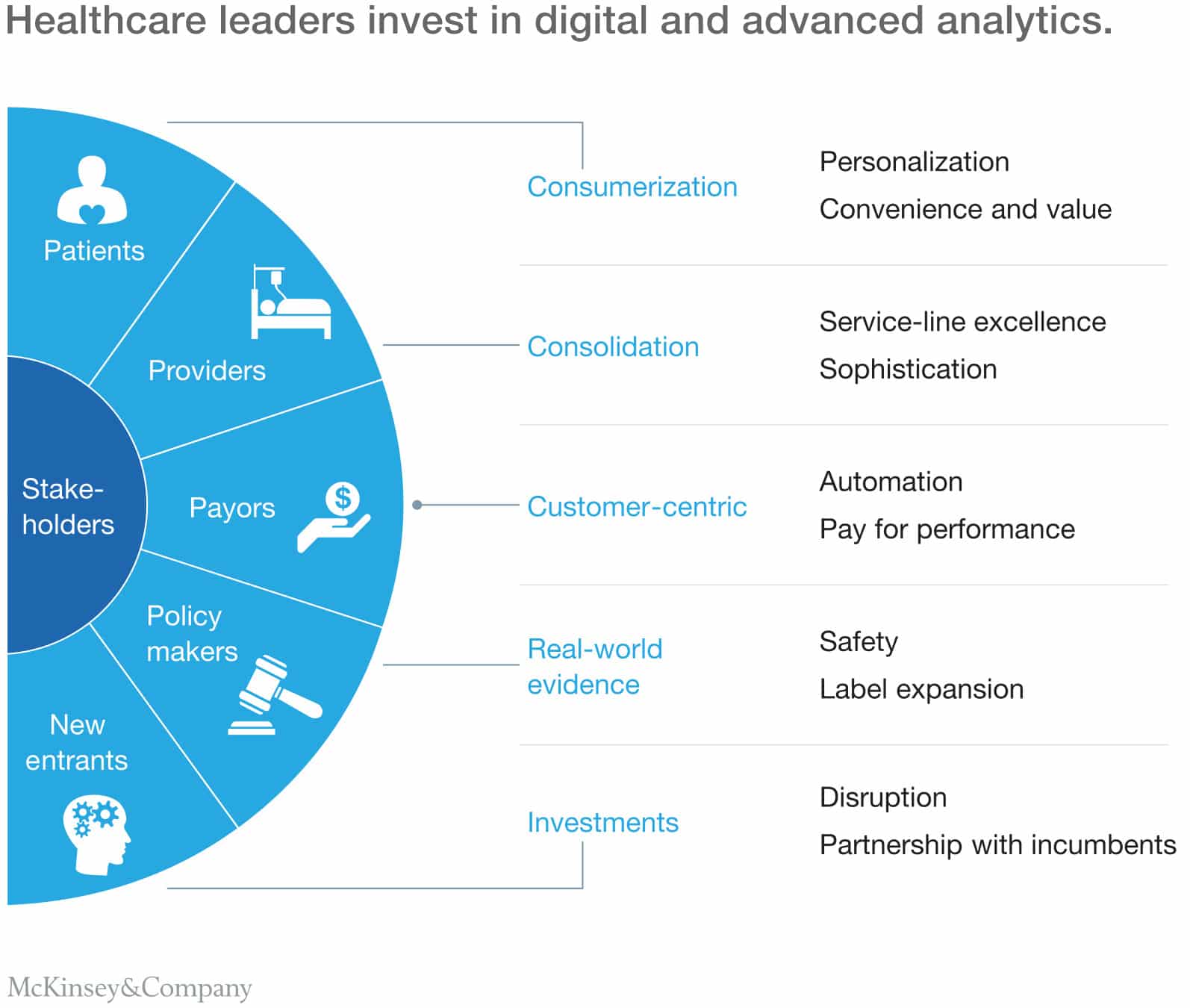
McKinsey Consulting takes an interesting perspective. Their research suggests that rather than leading top-level planning from an inward, company-first perspective, the key is to also keep an eye on stakeholders’ outlooks. Innovative advanced medical device companies focus on the user experience for all stakeholders, not just the end user. They also suggest leveraging technology to increase personalization while at the same time reducing costs and helping teams make decisions more quickly.
Innovative advanced medical device companies focus on the user experience for all stakeholders, not just the end user Share on XSome ways we’re seeing this play out are with the rise of non-invasive procedures, remote patient monitoring paired with end-to-end solutions, and collaboration across disciplines. Check out our five picks of companies pushing the envelope of advanced medical device innovation.
Ambu, Denmark
Founded in 1937, AMBU has seen decades of change in the medical device industry. Today, its focus is “single-use devices for inside and outside of hospitals.”
One product is a single-use endoscope that solves a persistent problem in endoscopy, namely the costs and risks of spreading infection by reusing endoscopes. Ambu also has products in neurology and cardiology, including electrodes and sensors.
Recent news for its pulmonology technology includes FDA clearances for its fifth-generation bronchoscope portfolio. They also sell products for airway management, anesthesia, and emergency care and training.
As a multi-national company, AMBU is aware of global sustainability challenges. The company’s sustainability strategies include a commitment to the 10 principles of the UN global compact and full support of UN sustainable development goals (SDG). This approach helps AMBU mitigate negative environmental impacts and manage the risk of environmental backlash against single-use devices.
Parasym Health, Estonia and UK
Founded in 2015, Parasym is a pioneer in the emerging electroceuticals sector. Electroceuticals can radically shift the treatment approach to heart failure with products that provide highly targeted treatment without drug interaction or side effects.
Heart failure patients are often on multiple medications not only for their condition but also to alleviate depression. All medications have potential side effects, including negative drug interactions.
Parasym develops innovative, neuromodulation treatments that are less invasive and not dependent on drugs. Reducing medication may significantly improve the quality of life for patients suffering from heart failure.
The Parasym device stimulates the vagus nerve and sends signals to the brain to calibrate the nervous system and reduce heart inflammation.
In 2022, the company released clinical trial results validating a non-invasive, drug-free option for people suffering from heart failure. The results showed that the patients who took part in the placebo-controlled study experienced measurable improvements. Patients using the device for an hour a day experienced a significant anti-inflammatory effect after as little as three months.
Bio-rithm / Femom, USA and Singapore
Pre-natal maternal care protocols have not changed significantly in decades. Bio-rithm’s current products emphasize devices that provide a continuum of care for pregnant women and empower clinicians with efficient tools to deliver quality care. This means monitoring maternal and fetal health more than just at a monthly appointment.
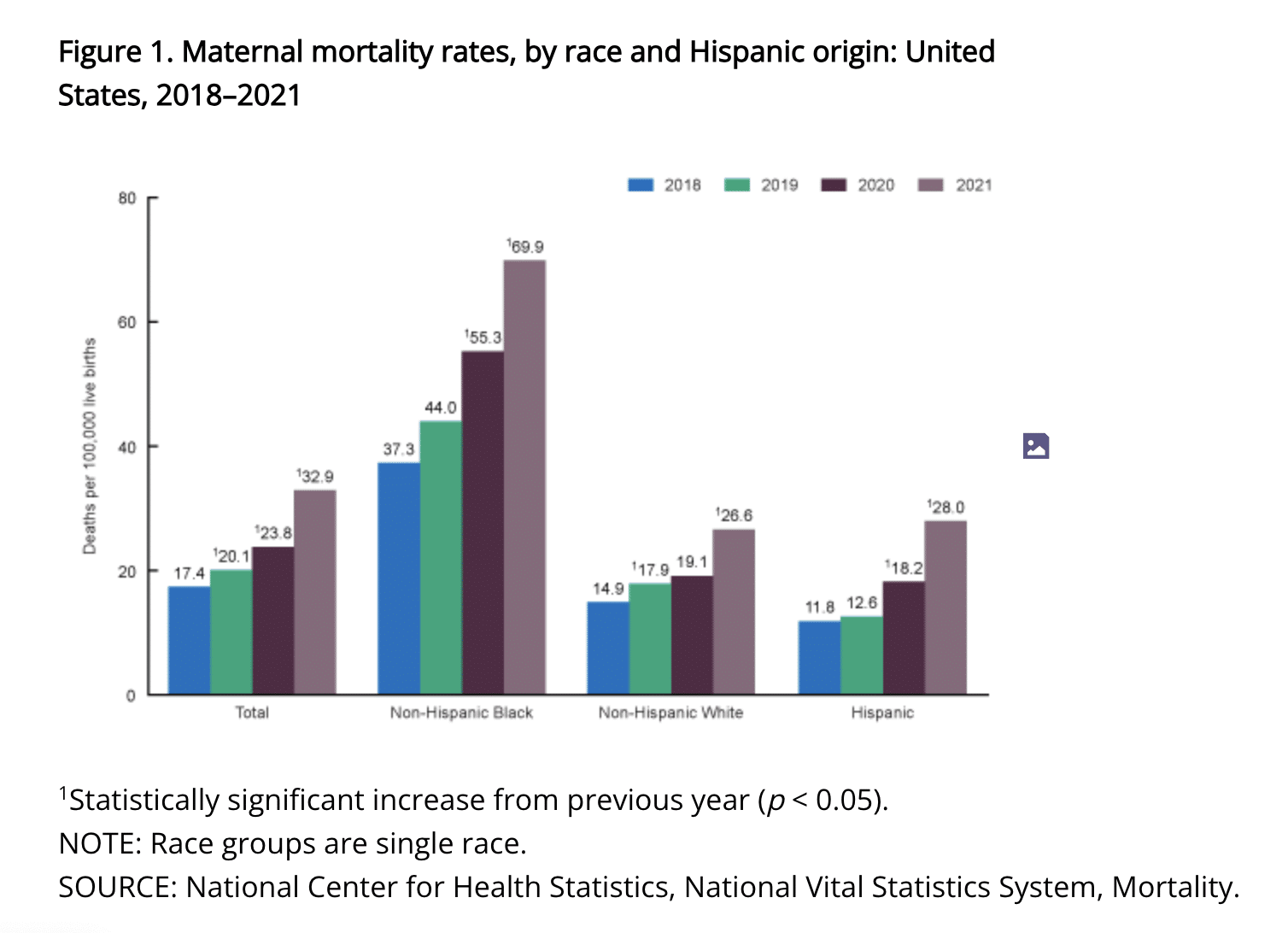
With maternal mortality rates increasing in the United States and many other countries, the need for a better approach to pregnancy and post-natal monitoring is clear.
The Femom product line is a comprehensive obstetrics remote monitoring platform to manage patients virtually using a combination of easy-to-use software and point-of-care devices to provide risk-based home care for prenatal and postnatal patients.
The Femom obstetric remote monitoring solution includes a clinical-grade fetal monitor and mobile application. The Femom fetal monitor is a non-invasive device measuring mother and child ECG, heart rates, mother’s uterine contractions, and more. Results are shared with PCPs on an ongoing basis, allowing for better decision-making not only during scheduled remote and in-person visits but in times of potential emergencies as well.
Vivos Therapeutics, USA
Multidisciplinary approaches seed medical device innovation in interesting ways. Vivos is a medical device company leveraging close collaboration between dentists and physicians to create new solutions for mild-to-moderate sleep disorders and dentofacial abnormalities.
The “Vivos Method” is a multidisciplinary treatment protocol that uses cost-effective oral appliance technology prescribed by trained dentists and medical professionals to treat dentofacial abnormalities and/or mild-to-moderate OSA. It’s noninvasive, nonsurgical, nonpharmaceutical, and virtually pain-free.
Vivos launched VivoScore Technology in 2021, a diagnostic sensor-ring recorder that assesses sleep quality. In January 2023, after an initial rejection by the FDA, Vivos secured FDA 510(k) clearance for its class II DNA oral appliance as a new treatment regimen for mild-to-moderate obstructive sleep apnea (OSA).
The DNA appliance treatment involves palate extension, tongue training to open the airway, and transitioning patients to breathing through the nose. The device can potentially improve sleep and quality of life for the estimated 30 million patients with mild to moderate OSA, currently relying on bulky CPAP machines that only address the symptom and don’t help patients transition to life without CPAPs.
Biotricity, USA
When you consider that heart disease accounts for about 20% of deaths in the US, it seems incredible that cardiac patients haven’t had the equivalent of a glucometer used by diabetics, to monitor their condition.
The founders of Biotricity thought so, too. The company’s broader positioning is as a medical technology company focusing on remote biometric monitoring and diagnostic and post-diagnostic solutions for chronic conditions and lifestyle improvement.
Biotricity is building out an integrated suite of offerings, including a suite of products that bridge industry verticals such as medical devices and telemedicine to help cardiologists provide personalized monitoring solutions. Innovations include a call-center-supported 24/7 electrocardiogram and a direct-to-consumer smartphone-enabled wearable EKG device for seamless user monitoring.
Its innovative suite of products and services is kind of like an unofficial case study for McKinsey’s advice to consider all stakeholders in the value stream. Biotricity’s approach offers stakeholders products and services that flexibly integrate different solutions in a unique, end-to-end solution approach.
Moving Ahead
The global pandemic accelerated several trends in health care directly tied to medical devices, such as remote monitoring, connected devices, and telehealth. AI is now expanding opportunities for personalization and improved decision-making. One of the biggest challenges to innovation in any field, of course, is regulatory compliance.
Are you an industry leader or startup looking to leverage trends and innovation for your company? At Galen, we are early adopters ourselves with years of experience helping our clients ride the waves of innovation while navigating regulatory riptides. Give us a call today if you have any questions.