Tech And Regulatory Trends for the Advanced Medical Device Industry In 2025
With a global pandemic and the meteoric rise of artificial intelligence (AI), history may view the first half of this decade as one of the most disruptive in the history of medtech. As we approach 2025, emerging technologies continue to impact device design and capabilities.
For founders and consultants in the medical device market, staying informed about tech and regulatory trends for the advanced medical device industry in 2025 is essential for maintaining a competitive edge.
This post explores the critical technological and regulatory trends shaping 2025, helping medical device leaders prepare for an innovative, compliant, and patient-centered future.
Emerging Technologies Transforming the Medical Device Industry
The medical device industry is experiencing a wave of technological breakthroughs. AI, wearable medical devices, and cloud computing innovations are leading the charge, all geared toward creating more connected medical devices and supporting precision medicine.
These technologies don’t just improve patient outcomes; they redefine the data capabilities, regulatory considerations, and safety measures medical device companies need to consider.
AI and Machine Learning
Artificial intelligence and machine learning are making significant strides in data analytics for medical devices. From analyzing complex patient data in real-time to providing predictive insights, AI enables more informed decision-making for healthcare providers and patients.
The surge in intelligent, data-driven insights accelerates the medical device development process, allowing companies to design devices that not only respond to patient needs but can also anticipate them.
Data privacy and data security remain central to integrating these technologies, especially as AI-powered devices rely heavily on continuous data collection and analysis.
Innovations in Wearable and Implantable Medical Devices
The demand for wearable devices and implantables continues to escalate. Devices like heart monitors, glucose sensors, and neurostimulators now offer remote monitoring options, giving patients and providers instant access to vital health information.
Wearable medical solutions support chronic condition management and enhance patient safety by enabling the early detection of health concerns.
As these devices generate real-time data, they integrate smoothly with cloud platforms that ensure data protection and regulatory compliance, positioning them as integral tools for remote patient care.
Advances in Telemedicine and Remote Monitoring
Following an exponential uptick during the global pandemic, digital health continues to gain traction. Remote patient monitoring and telemedicine solutions playing a crucial role in the post-pandemic healthcare landscape.
Medical devices that support virtual consultations and remote patient data collection offer more accessible care options and lessen the strain on healthcare systems. This trend aligns with regulatory priorities around patient data security and risk management, reinforcing the need for medical device companies to stay ahead of privacy protocols and industry trends prioritizing secure data handling.
R&D Focus Areas Driving Innovation
Major R&D trends are reshaping how companies approach device development, focusing on creating innovative, data-rich, and secure medical technologies. These developments span several exciting areas.
Key R&D Trends
Investment in nanotechnology, robotics, and augmented reality demonstrates the industry’s focus on highly specialized, patient-centered devices. These technologies enable more precise treatments and diagnostics, facilitating personalized medicine that meets individual patient needs.
Medical Device Patents and Innovation Trends
The rise in medical device patents reflects a growing commitment to innovation within the medical technology sector. Device companies are continually working to protect their proprietary developments, particularly in areas like remote monitoring and wearable devices.
As companies patent these innovations, they establish themselves as leaders in device technology, creating a competitive edge and fueling continuous advancements.
Collaborative R&D and Partnerships
Partnerships with tech companies, research institutions, and government bodies have become essential to medical device development. Collaborative R&D efforts reduce development costs, accelerate innovation, and result in devices that benefit patients and healthcare providers alike.
The Impact of Global Health Demands
The medical device market is shaped by pressing global health trends, including an aging population and the increasing prevalence of chronic diseases. Let’s take a closer look.
Aging Population and Rise in Chronic Diseases
The worldwide aging demographic creates greater demand for advanced medical devices tailored to age-related conditions. The charts below from Fidelity do a great job of illustrating the scale of global health demand.
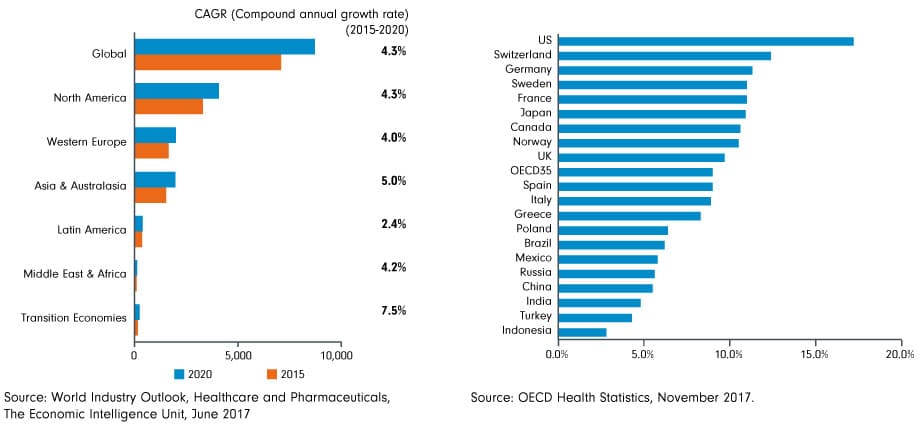
Chronic diseases like diabetes, cardiovascular disease, and respiratory illnesses require continuous management and monitoring. Remote, connected medical devices designed for these purposes provide patients with the tools to manage their health proactively while reducing the frequency of hospital visits.
Demand for Personalized and Precision Medicine
Advances in personalized medicine are transforming patient expectations, shifting toward treatments tailored to genetic and lifestyle factors. Medical devices now play a central role in precision medicine by delivering customized treatments based on individual patient profiles.
This trend has driven the development of devices that allow healthcare providers to implement personalized care strategies, thus improving patient safety and treatment outcomes.
The Role of Remote Device Technology in Global Health Challenges
Devices that support remote patient monitoring and data collection enhance healthcare accessibility, especially in regions with limited medical resources. These technologies also aid in tracking health data at scale, which is valuable for public health monitoring and preventive care.
Navigating the Shifting Regulatory Landscape
As technology reshapes the medical device industry, companies face new regulatory demands. Firms must proactively prepare for regulatory updates and adapt their processes to stay competitive and compliant.
Regulatory Changes
With advanced connected medical devices collecting and processing more patient data than ever, regulatory bodies like the FDA continue to update guidelines to prioritize data protection, data security, and patient safety.
Anticipated regulations may cover stricter data privacy standards, especially in response to new cybersecurity risks associated with remote monitoring and data collection.
Compliance Challenges
Navigating complex regulations while innovating in a fast-paced device market requires agility and a deep understanding of compliance obligations. With global markets introducing their standards, medical device companies must coordinate across regions to meet diverse regulatory requirements.
This complexity underscores the importance of building comprehensive compliance strategies that meet current standards and accommodate future regulatory shifts.
Strategies for Regulatory Adaptation
To successfully adapt to evolving regulatory landscapes, companies should invest in data privacy and data security practices and seek cloud platform solutions explicitly designed for medical technology.
Cloud platforms offer scalable and compliant environments for managing patient data, allowing companies to focus on medical device innovation without compromising security.
Partnering with cloud providers experienced in the medical sector, like Galen Data, helps companies maintain compliance while benefiting from cutting-edge data storage and analysis capabilities.
Moving Forward and Staying Competitive
Founders and consultants within the medical device market who stay informed and engage actively with industry trends position themselves to succeed in an increasingly competitive landscape. By prioritizing patient safety, data privacy, and effective risk management strategies, companies can navigate technological and regulatory shifts smoothly.
At Galen Data, we understand the unique challenges of managing medical device data in a secure, compliant, and scalable way. Partner with us to develop a robust data management strategy that empowers you to innovate while we handle the infrastructure.
Schedule a consultation with Galen Data today to explore how we can help your company securely store, manage, and analyze medical device data, ensuring you stay at the forefront of medical device innovation.