Top 5 Regulatory Trends in the Advanced Medical Device Industry
Innovation in medical devices is continuously evolving, driven by rapid technological advancements and a growing focus on patient safety and care quality.
As with all health care innovations, these advancements come with stringent regulatory requirements. Staying updated with these regulatory trends in the advanced medical device industry is crucial for companies looking to innovate while maintaining compliance.
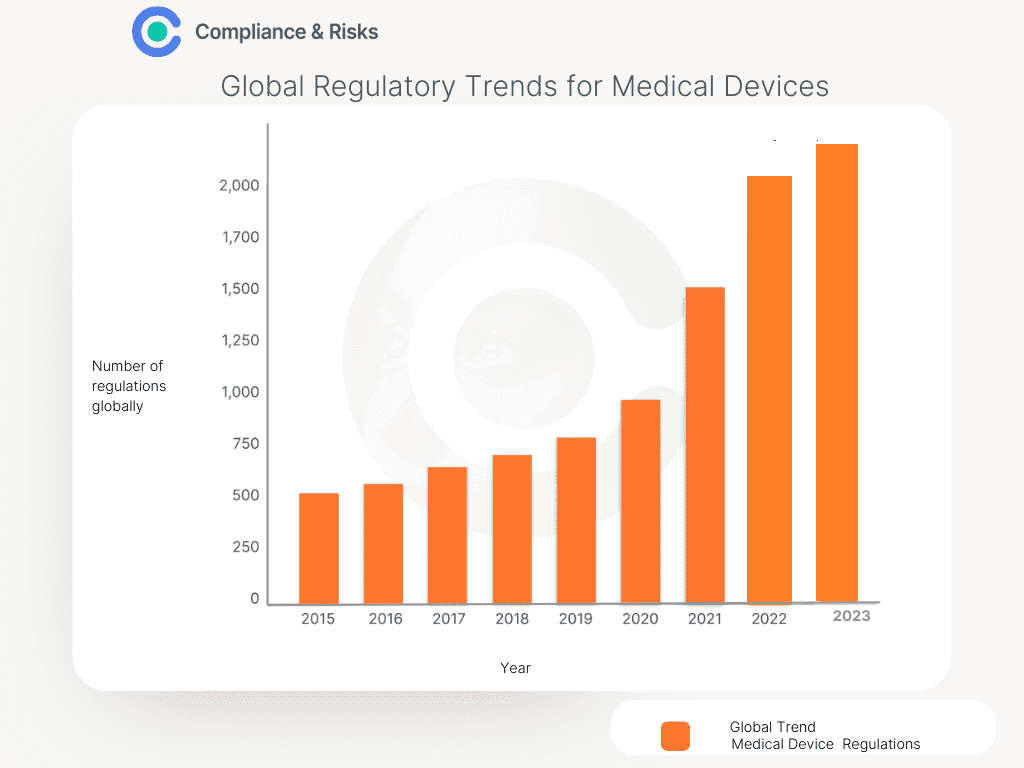
As complexity increases, so does regulation. The chart below shows the trend.
Galen Data, a leader in providing cloud solutions for the industry, understands the complexities of these regulations. This blog post will explore the top five regulatory trends shaping the medical device industry today.
Trend 1: Integration of Artificial Intelligence in Medical Devices
One of the most significant technology trends of our time is the integration of Artificial Intelligence (AI) and machine learning. AI is revolutionizing advanced medical devices by enhancing diagnostic accuracy, enabling predictive analytics, and improving patient outcomes.
AI-powered devices can analyze vast amounts of data quickly, identify patterns, and provide real-time insights that were previously unattainable.
Regulatory Considerations for AI in Medical Devices
Regulatory bodies are focusing on AI’s safe and effective integration. The FDA, for instance, has developed a framework for AI/ML-based medical devices, emphasizing the need for transparency, patient safety, and continuous learning systems.
Similarly, the European Union’s MDR includes provisions for AI, ensuring that AI-powered devices meet stringent safety and performance standards.
Challenges and Opportunities
The integration of AI presents both challenges and opportunities. Companies must ensure that their AI algorithms are validated, explainable, and compliant with regulatory standards. There is also a need for ongoing monitoring and updating of AI systems to maintain their accuracy and reliability.
Trend 2: Enhanced Post-Market Regulatory Trends in the Advanced Medical Device Industry
Trends in post-market regulation fall into two categories – market surveillance and data privacy. Let’s take a look.
Stricter Post-Market Surveillance Requirements
Post-market surveillance is a critical component in the lifecycle of medical devices, ensuring that stakeholders can promptly identify and address any issues or adverse events that occur after a device is on the market. Recent regulatory updates introduced stricter requirements for post-market surveillance, compelling medical device companies to adopt more comprehensive and proactive monitoring strategies.
Regulatory bodies, including the FDA, the EU MDR, and the European Medicines Agency (EMA), have emphasized the importance of ongoing surveillance to detect potential device malfunctions, safety concerns, and other issues that could impact patient health.
New medical device regulation guidelines mandate that companies must have robust systems for collecting, analyzing, and reporting post-market data. This includes adverse event reporting, trend analysis, and corrective actions when necessary.
To comply with these enhanced requirements, companies must integrate advanced monitoring and reporting mechanisms into their post-market surveillance processes. This involves leveraging real-time data collection and analytics to identify trends and issues promptly. Effective post-market surveillance ensures compliance and enhances patient safety and device reliability.
Data privacy and protection: one of the top regulatory trends in the advanced medical device industry
Data privacy and protection have become paramount concerns with the increasing digitization of healthcare and the proliferation of connected devices. Regulatory frameworks such as the Health Insurance Portability and Accountability Act (HIPAA) in the United States and the General Data Protection Regulation (GDPR) in Europe impose stringent requirements on how patient data is handled, stored, and protected.
Companies must ensure that their devices and data management practices comply with these regulations to protect patient privacy and avoid significant penalties. This involves implementing strong data encryption, secure access controls, and comprehensive data management policies. Companies must also ensure transparency with patients regarding how their data is used and obtain necessary consent.
Trend 3: Global Harmonization of Regulations
The medical device market operates globally, and the need for harmonized regulatory standards is increasingly critical. Regulatory harmonization refers to aligning regulatory requirements across different countries and regions to streamline the approval and commercialization of devices. Proponents of this trend desire to facilitate international trade, improve patient safety, and reduce the burden of regulatory compliance on medical device manufacturing companies.
Key initiatives like the International Medical Device Regulators Forum (IMDRF) and the Medical Device Single Audit Program (MDSAP) are promoting global regulatory harmonization. These initiatives aim to develop standardized guidelines and frameworks that can be adopted by multiple regulatory authorities, making it easier for companies to navigate regulatory requirements in different markets.
One significant challenge of regulatory harmonization is ensuring that products meet the diverse requirements of various jurisdictions while maintaining high safety and efficacy standards.
Companies must stay informed about international regulatory developments and be prepared to adapt their compliance strategies accordingly.
Trend 4: Evolution of Software as a Medical Device (SaMD)
Software as a Medical Device (SaMD) is software intended for medical purposes that is not part of a hardware device. The rise of digital health technologies has increased the development and deployment of SaMD, which includes applications for diagnostics, monitoring, and treatment of various medical conditions.
Regulatory bodies play a crucial role in acknowledging and addressing the unique challenges posed by SaMD. They have developed specific frameworks to guide its development, approval, and post-market monitoring.
For instance, the FDA has issued guidelines for SaMD, covering artificial intelligence and stressing the importance of demonstrating safety, efficacy, and performance. Similarly, the European Union’s MDR includes provisions specifically for SaMD, ensuring that these products meet rigorous safety and performance standards.
SaMD development necessitates a significant shift for companies towards a software-centric approach. This involves a focus on areas such as the software development lifecycle, risk management, and cybersecurity. Companies must ensure their SaMD products comply with relevant regulatory requirements throughout the product lifecycle, from initial design to post-market surveillance.
Trend 5: Increased Focus on Cybersecurity
With the exponential increase in connected medical devices with the Internet of Things (IoT), cybersecurity is a critical concern as medical devices are integrated into healthcare IT systems. The chart below of the projected healthcare IoT market gives some perspective to the magnitude of the overall issue.
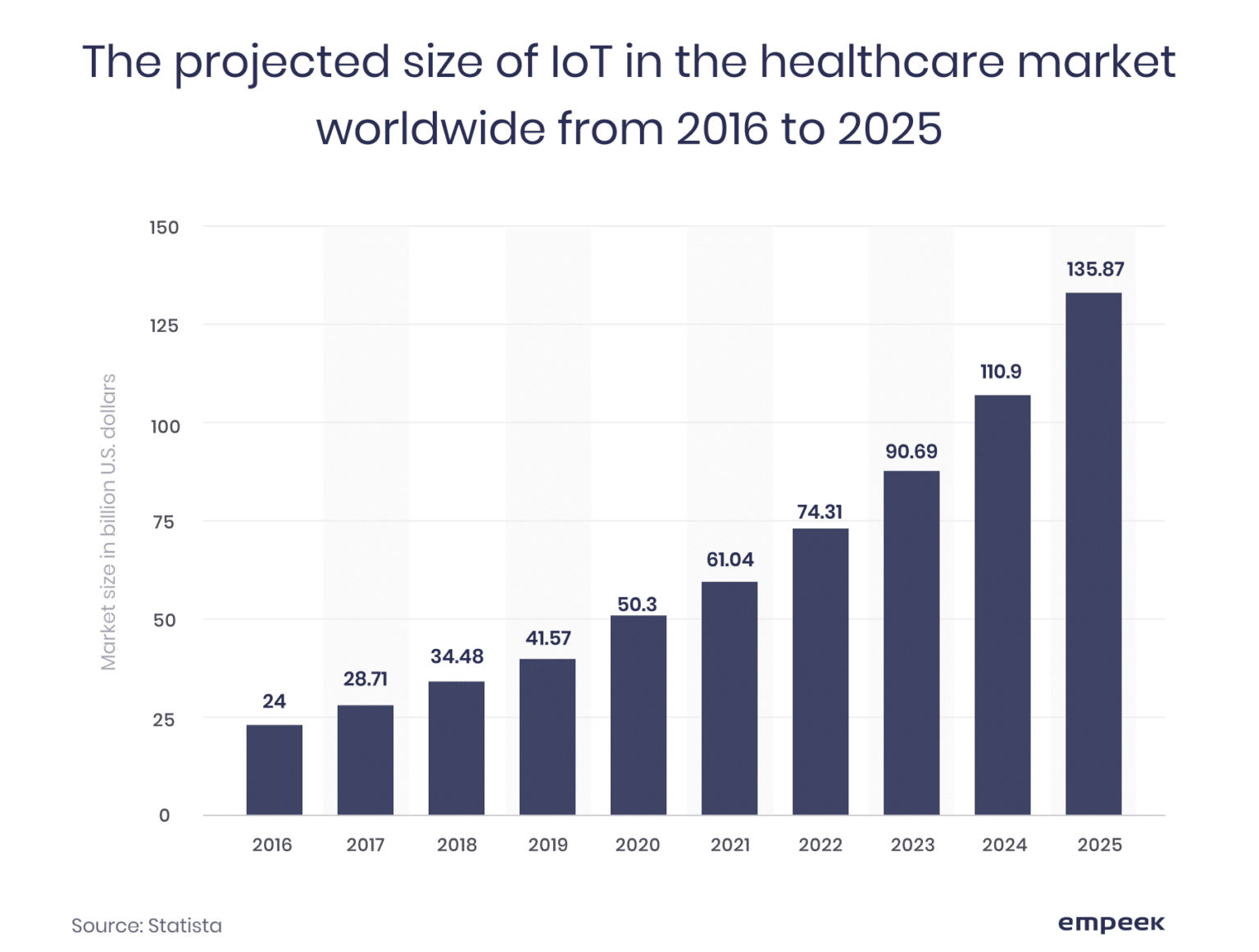
Cybersecurity threats pose significant risks to patient safety and privacy and to the overall functionality of medical devices.
Recognizing these risks, regulatory bodies have introduced stringent cybersecurity requirements to protect medical devices from potential cyber-attacks.
Recent regulatory updates have placed a strong emphasis on incorporating cybersecurity measures throughout the device lifecycle, from design and development to deployment and maintenance. For instance, the FDA has issued guidance documents outlining the necessary steps for ensuring cybersecurity in medical devices, including premarket submission requirements and postmarket management of cybersecurity vulnerabilities.
To stay compliant, medical device manufacturers must adopt a proactive approach to cybersecurity. This includes conducting thorough risk assessments, implementing robust security controls, and continuously monitoring for potential threats. Compliance with these regulations not only protects patients but also enhances the trust and reliability of the devices.
Moving Ahead
Staying ahead of regulatory trends is essential for medical device development companies looking to innovate while ensuring compliance and patient safety.
The top regulatory trends—cybersecurity, enhanced post-market regulatory demands, global harmonization of regulations, the evolution of SaMD, and the integration of AI—are critical areas that companies must address.
Galen Data provides a comprehensive cloud platform that helps medical device companies navigate these regulatory challenges. The Galen Cloud platform offers advanced security features, real-time data visualization and analysis, and compliance with multiple regulatory frameworks, enabling companies to focus on innovation while maintaining regulatory compliance.
For medical device companies seeking to stay ahead in the regulatory landscape, partnering with Galen Data can provide the tools and expertise needed to succeed. Contact Galen Data today to learn more about how their solutions can support your regulatory compliance efforts and drive medical innovation.