Update on Clinical Decision Support Systems
Technology is revolutionizing healthcare, yet the road to revolution can be bumpy at times. New technologies are blurring the lines between medical devices as gadgets and Software as Medical Device (SaMD). What’s more, healthcare providers face cognitive overload from device complexity and an increase in patient data. At the same time, the demand for individualized patient care is growing.
Clinical Decision Support Systems (CDSS) are a valuable tool that addresses these challenges, empowering healthcare professionals with timely, evidence-based decision support and contributing to improved healthcare outcomes.
CDSS and advanced medical devices form a symbiotic relationship in healthcare. Share on XCDSS enhance the capabilities of advanced medical device by providing real-time decision support, analyzing data, ensuring safety measures, and paving the way for future advancements.
By integrating CDSS into advanced medical devices, manufacturers can enhance patient care, improve healthcare outcomes, and optimize the use of their devices. This post explores more about CDSS and also take a look at their FDA trajectory.
Integration of CDSS with Advanced Medical Devices Improves Patient Care
CDSS enhance medical device capabilities by seamlessly integrating with devices to create valuable feedback. For instance, CDSS in a smart infusion pump can provide dosage recommendations and flag potential drug interactions. Similarly, CDSS can enhance the capabilities of implantable devices, such as pacemakers, by continuously monitoring patient data and providing real-time alerts in case of irregularities.
Another critical advantage of integrating CDSS with advanced medical device is the provision of real-time decision support. For example, in a surgical robot system, CDSS can analyze patient-specific data during a procedure and provide surgeons with recommendations on the optimal surgical approach, reducing human error and improving surgical outcomes. CDSS can also assist in monitoring vital signs during anesthesia administration, alerting healthcare providers to potential complications.
Data Analysis with CDSS and Advanced Medical Devices
In the aggregate, advanced medical devices generate vast amounts of patient data over time. To optimize patient care, providers need a way to capitalize on the data, sometimes almost instantly, for better patient outcomes.
CDSS utilize artificial intelligence and machine learning algorithms to analyze this data, identify patterns, and make informed predictions. CDSS also enable advanced medical devices to detect subtle changes and patterns in patient data that might go unnoticed by healthcare professionals.
By leveraging historical data, CDSS can provide insights and suggest changes in treatment plans or device settings to optimize patient care. For example, an implantable cardiac device integrated with CDSS can identify trends in cardiac function, triggering automatic adjustments or alerting healthcare providers to potential complications.
Safety and Effectiveness of advanced medical devices with CDSS
CDSS play a vital role in advanced medical device safety performance. CDSS can detect deviations from expected values or standards by continuously monitoring device performance and patient data. This prompts the system to issue alerts and reminders, preventing potential adverse events. For example, CDSS integrated into a diagnostic imaging device can flag improper positioning or incorrect settings, minimizing the risk of misdiagnosis.
CDSS are a reliable companion for healthcare providers, providing them with evidence-based recommendations, treatment guidelines, and reminders for necessary actions. This assistance significantly reduces the cognitive load on healthcare professionals, minimizes errors, and promotes adherence to best practices.
FDA CDSS Guidance and Regulation
The U.S. Food and Drug Administration (FDA) has issued several guidance documents related to CDSS. These guidelines clarify the regulatory requirements and expectations for developing, validating, and using CDSS in healthcare settings. The September 2022 guidance document can be downloaded from the FDA’s website.
Here is a brief timeline of a few key FDA guidance documents related to CDSS:
- 2017 – “Clinical Decision Support Software”: This guidance document outlines the FDA’s approach to regulating software that provides clinical decision support. It clarifies the types of software functionalities that the FDA considers regulated medical devices and those that are not.
- 2019 – “Policy for Device Software Functions and Mobile Medical Applications”: This guidance document clarifies the FDA’s approach to regulating software functions intended to be used with medical devices, including CDSS. It distinguishes between functions that meet the definition of a medical device and those that do not.
- 2019 – “Clinical Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff”: This draft guidance provides recommendations for developing and clinically validating CDSS. It addresses the need for a rigorous software development lifecycle, including proper testing and validation procedures.
- 2019 – “Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD): Draft Guidance for Industry and Food and Drug Administration Staff” (2019): While not specific to CDSS, this draft guidance provides information on the regulatory considerations for medical software that incorporates artificial intelligence and machine learning algorithms, which CDSS often utilizes.
2022 FDA update – CDS Software or Medical Device?
Under the 2016 21st Century Cures Act, Congress created the CDS umbrella to exempt certain kinds of software from device regulations.
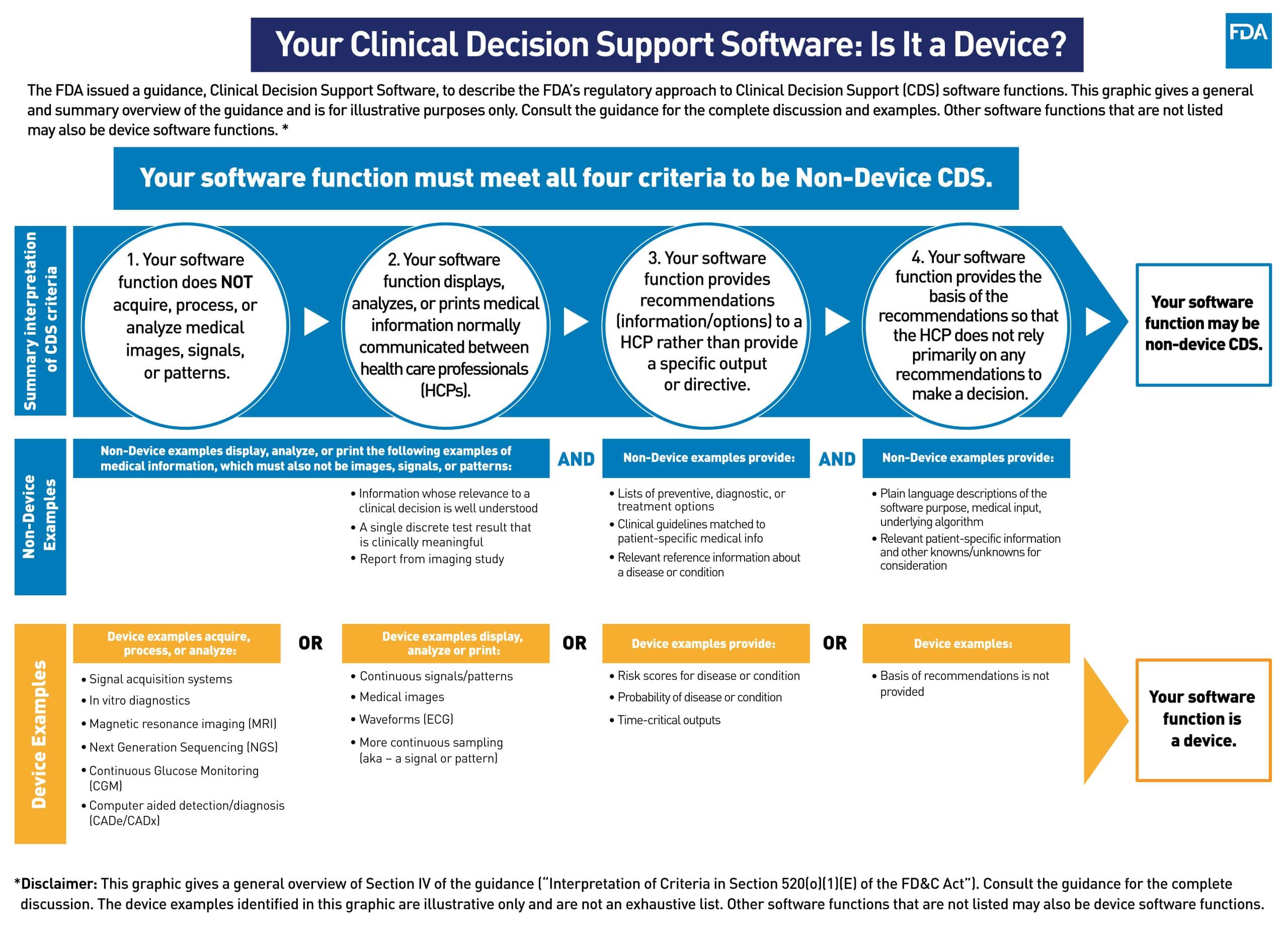
In September 2022, the FDA issued final guidance identifying four criteria that a software must meet to qualify as CDS software and be exempt from device regulations. The criteria are
- Not intended to acquire, process, or analyze medical images, signals, or a patterns
- Intended to display, analyze, or print medical information
- Intended to provide or support health care recommendations such as diagnosis, treatment decision, or condition
- But do so in a way that an HCP can independently review or verify how those recommendations were provided
While the criteria may seem to be straightforward, there is some controversy around these criteria that perhaps the FDA went beyond the spirit (and some argue even the letter) of the law.
The criteria represent a deviation from the draft guidance and potentially exclude more software systems (specifically quite a few of AI or ML-based systems) from being recognized as CDS software.
The requirement above distinguishes what FDA defines as routine medical information (information used today) from signals and patterns. e.g., a routine blood pressure reading in the clinic, is considered medical information, while a continuous blood pressure reading will be considered a signal. Also, recommendation systems that don’t disclose the method in the public domain or use what is already known to be relevant, may not be included in the CDS umbrella.
For further insight about the FDA guidelines, check out this episode of the Global Medical Device Podcast – Why FDA is Prioritizing Clinical Decision Support Software & Why You Should Care.
At Galen Data, we are early movers in the SaMD space, developing our own unique, secure cloud-based modular platform for research, reporting, and compliance. Do you have questions about the 2022 guidance or any other topics around CDSS for your medical device? Feel free to reach out to our team, we’re happy to help.