Designing for Compliance: QMS & Validation Testing for SaMD vs. Physical Devices
“God is in the details” is an old German proverb that Nietzsche (not surprisingly) paraphrased to the more common saying “the Devil is in the details.” While Nietzsche knew nothing about medical device regulatory compliance and testing, anyone experiencing the journey to FDA approval would agree that the process often feels more devilish than divine.
In medical device compliance, the keys are planning your Quality Management System (QMS) from the start and updating the QMS for the project’s entire life cycle. In other words, your QMS is a constant companion that tracks your progress, not a one-and-done plan that sits on a virtual shelf.
Your QMS is a constant companion that tracks your progress, not a one-and-done plan that sits on a virtual shelf. Share on XInnovation in software as a medical device (SaMD) also affects the compliance journey. Though compliance strategies for all devices share some common ground, differences exist between hardware and software medical devices. This post provides an overview of the QMS process. For a deep dive, you can check out this webinar on YouTube that goes into much more detail.
Building a Robust Foundation: Quality Management Systems
QMS is the regulatory compliance cornerstone of medical device development. The QMS establishes a structured framework ensuring device safety, efficacy, and compliance. Whether dealing with hardware or software, a robust QMS is your compass.
Planning the QMS can feel a bit overwhelming. Think of it as going slow at first to be more efficient later. Companies with solid plans can avoid delays in the long run.
For example, if the FDA ever audits your process, a QMS will pay for itself many times over. Kyle Rose, President of Rook Quality Systems, told us he had seen cases where the FDA orders devices without proper QMS off the market or even mandates their destruction. It wasn’t the lack of a QMS per se that dealt a death blow; it was the fact that the FDA couldn’t find the information they needed to justify giving the device a pass.
Below are the elements of a superior QMS.
- Quality Manual – A comprehensive document outlining system scope, market jurisdictions, and exclusions from standards.
- Document Control – Managing documentation efficiently through an EQMS (Electronic Quality Management System).
- Training – Equipping personnel with a deep understanding of the quality system.
- Design Control and Risk Management – Procedural guidelines for design and development, adhering to standards and risk management.
- Supplier Assessment: Ensuring supplier alignment with quality requirements and timely notifications. Supplier assessment deserves careful attention throughout your design process because if there is an issue with your device because of a supplier, the FDA still holds you responsible.
Navigating Design Control and Validation
The design control process forms the core of medical device development, mapping the journey from user needs to validated outputs. This process is pivotal for both hardware and software devices.
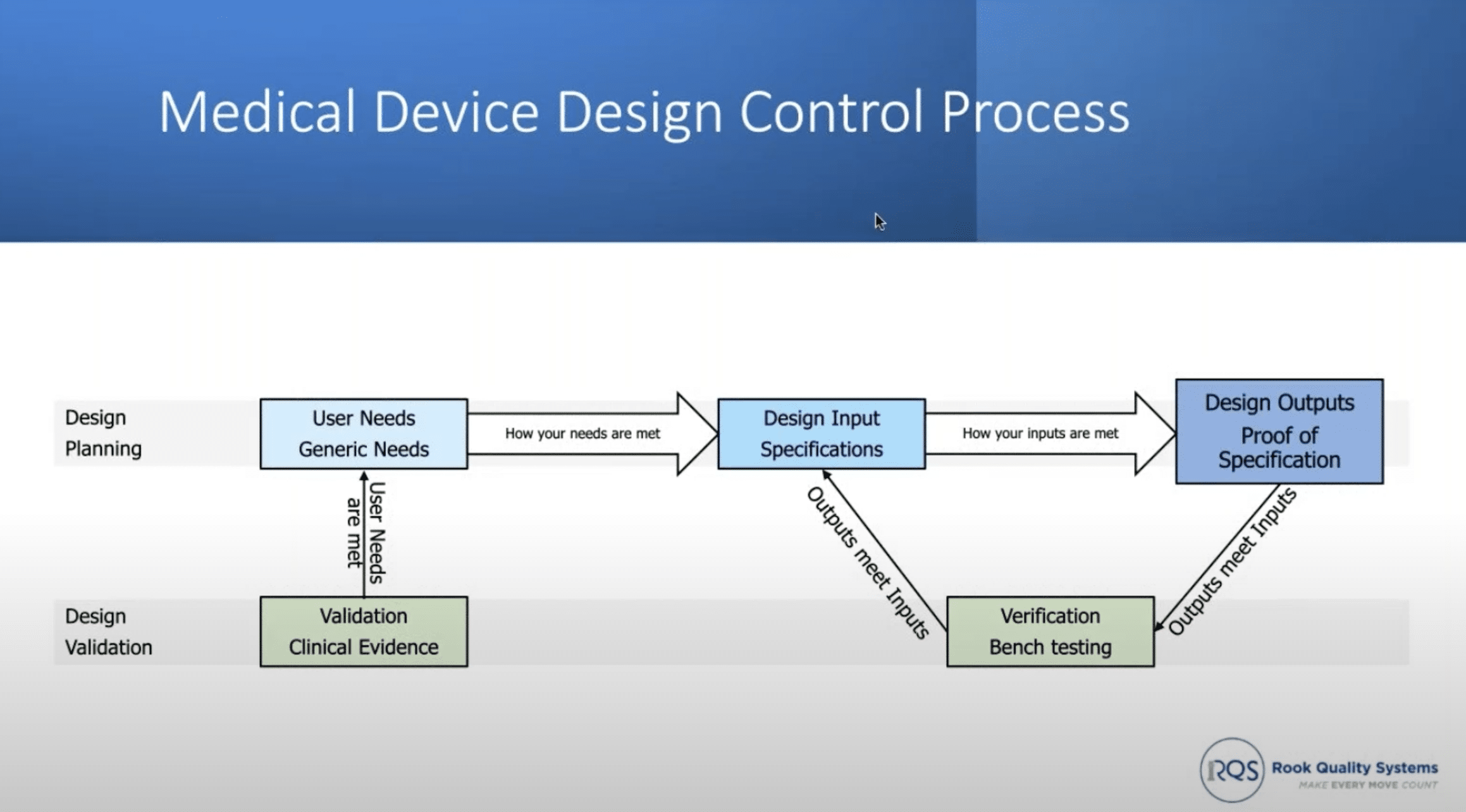
Source: Rook Quality Systems
The Design Control Phases include the following elements:
- User Needs – Identifying high-level safety, accessibility, and usability requirements.
- Design Inputs – Detailing specifications like size, accuracy, and performance.
- Design Outputs – Transforming inputs into tangible components, encompassing drawings, schematics, and software code.
- Verification – Rigorous testing to confirm adherence to specifications.
- Validation – Establishing clinical evidence and usability through user testing
The Design Control Traceability Matrix pulls all the information above together into one exhaustive document linking user needs, design inputs, outputs, and verification/validation requirements.
Medical Device Testing: Hardware vs. Software (SaMD)
The key differences between hardware and software devices are the complexity and time required for testing. Software as a medical device (SaMD) is a digital realm for medical applications like diagnosis, treatment, and management. The intangible nature of SaMD means that tests can be validated via virtual simulations and usability studies.
Hardware devices require:
- physical devices to be made to specification,
- supplier approval incoming inspection in assembly trial and error,
- specific amounts of devices to be made for testing.
They also may require equipment calibration validation for manufacturing and testing and shelf life shipping sterilization and external testing
In contrast to hardware devices, SaMDs are:
- much faster and cheaper to test,
- easier to fix edit updates,
- harder to come to a design and feature freeze.
Even though the process differs, SaMD still requires planning and testing documentation.
Blueprint for Hardware Testing
Here are some elements to consider when planning for hardware testing.
1. Test Plotting – Chart clear test objectives and foresee the path ahead. Define the device’s testing scope, sample size, and configuration, mirroring real-world scenarios.
2. Protocols: Define test protocols, device quantity, testing methodologies, and acceptance criteria. Confirm pass/fail benchmarks beforehand.
3. Set up a system for tracking and analyzing data.
4. Documentation – Chronicle every testing stage, protocols, results, and any design alterations.
5. Integrate results and document any retest as required.
Software as a Medical Device (SaMD) Testing and Validation
Apart from overall device safety and efficacy, cybersecurity is probably the biggest concern of the FDA (and your end users). Another difference from traditional medical devices is the need for a Software Bill of Materials (SBOM), which is a thorough documentation of software components, libraries, and dependencies.
Recommended stages for SaMD include:
- Software development schedule – create phases and “sprints” from lean development methodology.
- Create software design process – code requirements, platform and architecture description and diagram.
- Software implementation – test implementation progress and fix bugs during the implementation phase
- Software testing and software maintenance
Moving Ahead
The QMS is a mission-critical part of a successful FDA approval process because it provides a detailed framework that tracks the progress of your device development. Do you have questions about building a QMS for your project? Reach out today to get the conversation started.