Medical Device Product Development: 5 Essential Steps to Bring Your Device to Market
People often view successful innovation as kind of like achieving “overnight success”. In reality, successful teams bringing innovation to the marketplace know that the process is a long, challenging marathon of problem-solving and collaboration.
Of all the fields that work with emerging technologies, advanced medical device product development has one of the most complex journeys. Medical device development requires technical innovation and a deep understanding of regulatory requirements, patient safety, and market needs.
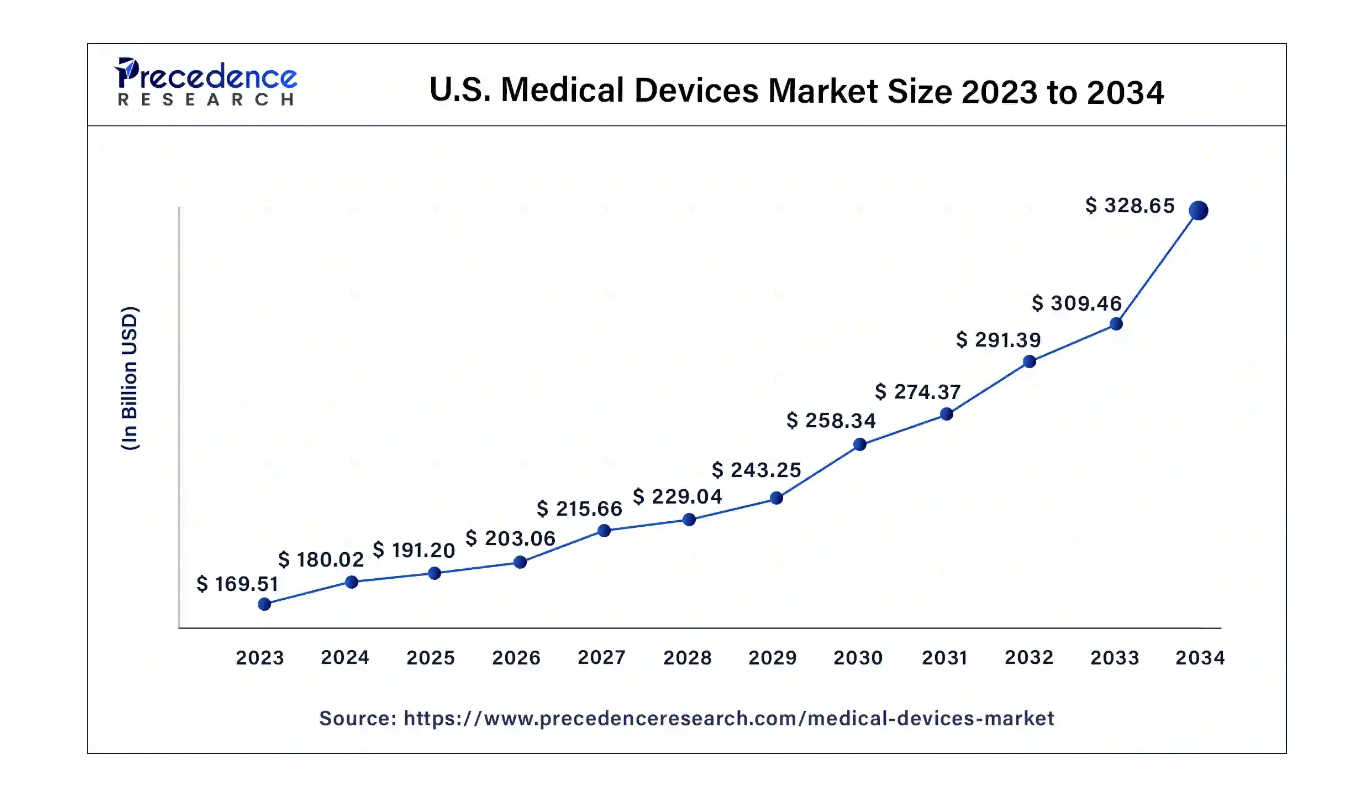
In spite of regulatory hurdles, strong projected growth in the medical device market size fuels continued innovation and investment. The United States dominates the medical devices market, which is predicted to grow at a CAGR of 6.2% from 2024 to 2034.
For founders of medical device companies and consultants guiding them through this process, knowing the proper steps can make the difference between successfully bringing a product to market or facing costly delays.
Efficient and secure data management is one critical component that can streamline this journey. As digital solutions transform the industry, cloud platforms such as Galen Data are pivotal in supporting the development and compliance process.
In this guide, we’ll walk you through five essential steps of advanced medical device product development and highlight how leveraging the right tools can bring your product to market smoothly.
Step 1: Product Development Concept and Feasibility
The first step in the medical device development process is turning your initial concept into something tangible. This phase involves assessing the technical and commercial feasibility of the medical device you plan to develop. Key activities include market research, identifying unmet clinical needs, and determining whether your idea is viable from both a functional and regulatory standpoint.
During this stage, comprehensive market research is critical. Understanding the competitive landscape, the potential demand for your device, and any existing solutions will help refine your concept. Equally important is conducting feasibility studies to evaluate whether your device can be realistically designed, developed, and manufactured. Feasibility isn’t just about innovation; it’s about balancing that innovation with practicality.
Data plays a crucial role during this phase. As you gather feedback from healthcare professionals and potential users, it’s essential to have a reliable platform to store and analyze this information securely. Cloud based platforms offer secure and scalable data management that allows you to organize feasibility data in a compliant manner. With built-in scalability, you can collect extensive feedback and refine your device concept based on real-world insights without worrying about data loss or security breaches.
Step 2: Design and Development Plan
Once your concept is validated, the next step is designing and developing your medical device. This stage involves turning your idea into a functional prototype and ensuring that it meets the necessary safety and performance standards. The design process includes everything from system architecture and component selection to risk management and usability testing.
During this stage, it’s crucial to implement design controls to manage risks, ensure compliance, and guarantee that the device functions as intended. Design controls outline how you document each design phase, from initial sketches to final specifications, ensuring your development aligns with regulatory requirements. Effective collaboration between teams—engineers, regulatory specialists, and quality assurance professionals—is key to refining and optimizing the design.
Artificial intelligence (AI) is also transforming this stage by accelerating design iterations, improving risk assessments, and assisting in optimizing device functionality. AI-driven predictive analytics can analyze vast amounts of data in real-time, identifying potential design flaws or performance issues earlier in the process. This not only reduces costly redesigns but also enhances overall device safety and efficiency.
This is where a robust cloud platform like Galen Data becomes invaluable. Whether you need to share updates with regulatory bodies or provide real-time data access to your internal teams, a compliant, secure cloud solution supports you every step of the way.
Step 3: Verification and Validation
After the design phase, your device must undergo rigorous verification and validation (V&V) processes to ensure it functions as expected and meets all regulatory requirements. Verification is confirming that the design outputs match the design inputs—essentially, ensuring the device was built correctly according to specifications. Validation, on the other hand, ensures that the device performs as intended in the real world for its intended use.
The V&V process involves various tests, including mechanical, electrical, and software testing, depending on the nature of the device. In addition, usability testing ensures that the device is user-friendly, particularly for patients or healthcare professionals. It’s vital to conduct these tests in a controlled, documented environment to ensure that the results can be reviewed by regulatory agencies such as the FDA.
Step 4: Regulatory Approval and Compliance
One of the most challenging medical device development steps is navigating complex regulations to achieve FDA approval. Regulatory bodies like the FDA in the U.S. and other global agencies have stringent standards for medical devices to ensure patient safety and device effectiveness.
Depending on the type of device you’re developing, you may need to go through different approval pathways based on FDA regulations, from premarket submission to post-market surveillance. Also be aware that risk is a major factor in a device’s regulatory pathway. From Class I, Class II and Class III, it makes sense that device development for higher risk profiles face more stringent regulatory hurdles.
Regulatory approval requires extensive documentation to prove your device meets safety and performance criteria. This includes providing clinical data, detailed device descriptions, and evidence from your verification and validation processes. The process doesn’t end with the approval, though—once your device hits the market, you must maintain compliance by continuously monitoring its performance, reporting adverse events, and ensuring quality control.
Step 5: Manufacturing and Commercialization
Once your device receives regulatory approval, the final step is scaling up for manufacturing and commercialization. This stage involves working with manufacturers to mass-produce your device, ensuring that each unit meets the same safety and efficacy standards as your prototypes. This step also includes creating a go-to-market strategy, which involves marketing, distribution, and sales channels to ensure your device reaches the right healthcare providers and patients.
It’s crucial to maintain stringent quality control processes during manufacturing to ensure every unit is consistent. You’ll also need to track performance post-market to make any necessary improvements based on real-world data. At this stage, having a secure data management system like Galen Data’s platform can streamline the collection and analysis of post-market data, ensuring that your device remains compliant and performs effectively.
Common Pitfalls and How to Avoid Them
Medical device commercialization is a lengthy and challenging process, with many potential obstacles along the way. Some common pitfalls include underestimating the regulatory requirements, neglecting robust verification and validation, and failing to manage data effectively throughout development.
To avoid these setbacks, it’s critical to have a detailed plan in place for each development stage. Partnering with a cloud data management platform like Galen Data can be a game-changer, especially for companies lacking in-house regulatory expertise or scalable infrastructure. Galen Data’s platform helps minimize compliance risks, provides secure storage for sensitive data, and supports collaboration between teams across various development phases.
Additionally, maintaining a clear focus on FDA and global regulatory requirements throughout the development process can save time and resources, ensuring smoother approval timelines.
Moving Ahead with Advanced Medical Device Product Development
As medical device product development grows more complex, having the right data management partner can be the key to success. Galen Data provides a comprehensive cloud platform that simplifies the development process, from medical device design and testing to regulatory approval and device commercialization. Partner with Galen Data to:
- Develop a secure and scalable data management plan.
- Leverage our expertise in medical device data and compliance.
- Focus on innovation while we handle the infrastructure.
Schedule a call with us today to discuss your specific needs and see how Galen Data can help you store, manage, and secure your medical device data at scale.