Beyond the Tech: Crucial Keys for Medical Device Startup Success in the US Healthcare Market
Innovative technology is the most important predictor of an advanced medical device startup’s success, right?
Or is it?
Kind of like the kid in the class voted “most likely to succeed” who never quite realized her potential, even a revolutionary medical device technology won’t see the light of day without the proper startup ecosystem.
In the realm of startup complexity, medical device company startups are at the top of the list, mostly because of regulatory scrutiny.
Startups must satisfy several key stakeholder groups in parallel and implement ironclad documentation and project management protocols. Click To Tweet Adding to the challenge is investor pressure to get to market as quickly as possible while resisting the temptation to rush crucial testing phases necessary for gaining regulatory approval.
Let’s look at the stakeholders involved and a broad overview of the phases for advanced medical device development.
Stakeholders for Medical Device Startups
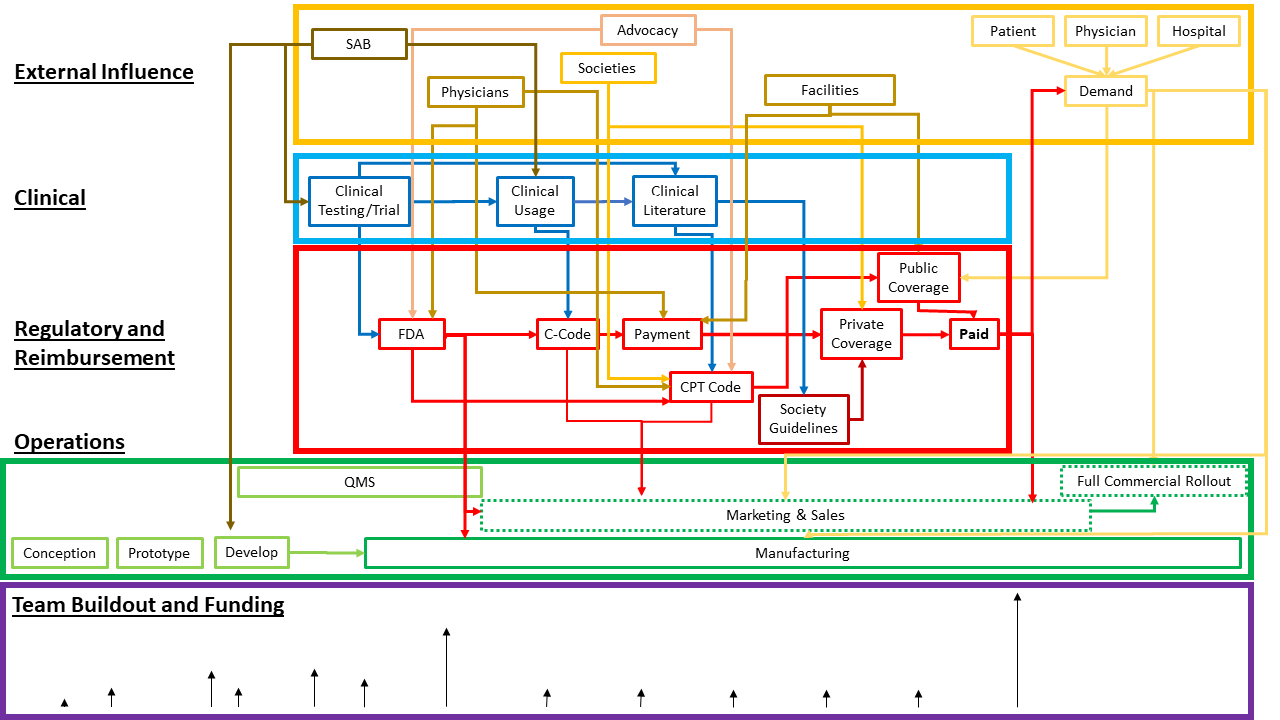
Founders, investors, development team, management team, government, insurers, customers, and end-users are the primary stakeholders for an advanced medical device startup. Each actor has their own perspective and goals. The graphic below shows an example of how stakeholder interests overlap with device development and timing.
A successful medical device startup starts with the founders’ idea, supported by a phased build-out from the development team.
Before they can hire technical talent, founders often need to court investors. Investors look for innovative devices that meet an unaddressed need. If your device requires clinical trials, you will need investors that understand the development lifecycle will require high cash flow before FDA approval and launch.
The average cost to bring a 510(k) product from concept to market is over $31 million. About 77% percent of the cost is for regulatory and FDA-related activities. The regulatory timeline can also affect medical device company valuations, too.
Depending upon their skill set, the founders of an advanced medical device startup may not have a business background or proficiency. While prototyping the device technology is the priority, the initial team needs to consider marketing and regulatory compliance very early on in the process. A critical part of pre-development is market feasibility analysis.
Companies need to plan phased builds for marketing, strategic partnerships, sales, and admin support that are critical to launching and sales growth. If the paying customer is a health care provider or HDO, you need to consider the end user – your customer’s customer – in your marketing mix as well. Providers and patients have very different user journeys and the messaging needs to be tailored to each segment.
Project complexity and reporting requirements to achieve regulatory approval necessitate Quality management systems and project documentation at every stage of the process. Bottom line: documentation is especially crucial for medical device startups and founders should establish a system very early and be sure it does not become an afterthought for busy teams, to be caught up on later.
Achieving FDA or other regulatory approval is a major milestone. Creating a strategy for risk management, regulatory strategy, product design, validation plans, post-launch surveillance, and quality control starts in phase one and lasts for as long as the product is on the market.
The other government-related consideration is will your device operate within the Medicare ecosystem, and if so, how does this affect your reimbursement strategy? This overlaps with the insurer stakeholder group as well.
Below is a basic overview of the phasing of the advanced medical device startup process.
Phases for Medical Device Startups
Phase I – Foundation and Risk Analysis
The purpose of Phase 1 is to confirm there is a market for your device and that the project is financially feasible. Points to consider include:
- Establish a Quality Management System to manage procedures and forms of how you will document and communicate your activity and progress. Most medical device companies adopt the ISO 13485 standard. Check out our Guide to ISO 13485 for startups here.
- Create a preliminary funding strategy.
- Marketing research – who is your competition? How will you solve problems differently? Will you have one primary paying customer or two customers – the patient and the healthcare provider or HDO?
- Will you need Clinical trials for your product? Clinical trials add extra expense, affecting time to market and the types of investors you will attract.
- Establish a Quality Management System to manage procedures and forms of how you will document and communicate your activity and progress. Most medical device companies adopt the ISO 13485 standard. Check out our Guide to ISO 13485 for startups here.
Phase 2- Concept Development and Feasibility
In phase 2 you develop the concept and prove it works. Considerations for phase 2 include:
- Pursue funding and planning for building a prototype.
- Continue market research: begin surveying customers and gathering more data about competition. Be flexible as you may need to adjust the original design based on new findings.
- Pre-planning for product launch timeline – specific factors affecting timing include the level of innovation and price point.
- Review the regulatory requirements from the very beginning to estimate the time needed for development, testing, and pre-market submission to FDA. New untested tech may require 18 months of lead time, whereas less expensive investments or devices based on existing approved functionalities may need only 3 to 6 months to launch.
Phase 3 – Design Development, Verification, and Validation
In Phase 3 you build out the medical device, and the teams begin validation and verification of your device. Considerations include
- Thorough understanding and plan for performing all the necessary regulatory clinical trials and tests for any market where you will sell your device. You don’t want to get to Phase 4 and realize you haven’t performed some necessary tests.
- Review your risk management considerations at this stage, too. The FDA regulations are in place to increase patient safety and minimize risk. While it will not be the most enjoyable part of the development process, it’s important to consider as many risk scenarios as possible, from mild to worst case, in terms of risk to patient safety.
- Obviously, companies cannot sell technology that is pending FDA approval. However, the sales team may begin establishing relationships and having preliminary conversations before the official product launch.
Phase IV – Final Validation and Product Launch Preparation
Once you clear the final validation hurdle, it’s time to complete the technical documentation and also turn on the marketing engine. FDA approval clears the way for sales activity in the US market. Companies outside the EU who want to enter European markets will need an Authorized Representative to represent their business in Europe.
Other launch preparation activities include:
- Finalize design transfer
- Decide on how the company will distribute the device
- Register device
- Create a marketing plan
- Create a reimbursement strategy
- Collect, create and organize all the literature, videos, graphics, illustrations, or content assets that will attract and engage future customers
- Provide training for your sales team
- Strengthen post-launch service and help desk support
- Create post-launch performance data gathering plan
Phase V – Product Launch and Post Launch Assessment
By this time in the process, founders may feel that the ecosystem resources are strained to the limit. Dig deep, affirm your teams, and consider the following:
- Run a final check of your technical documentation for updates
- Risk management: create and test a system to capture complaints and loop them back into your risk management system
- Be sure that your sales or marketing team has a post-sale process for staying in touch with customers. Continue to survey customers about their experience using the product.
- Revise customer-facing documentation as needed
- Adjust and strengthen sales and marketing materials and processes based on customer and end-user feedback. Create case studies and gather testimonials for example.
Moving Ahead
From concept to market, founders may feel like their startup is a growing cyclone of moving parts. The main points to remember are to start early, don’t skip steps or take shortcuts, and document everything. Check out our additional resources for more details to help you stay calm in the center of the storm.
The right partners are crucial to your success. Galen Data’s cloud platform provides a cost-effective and compliant solution for medical device connectivity, data visualization, analytics, and much more. Contact us today to discuss your project.