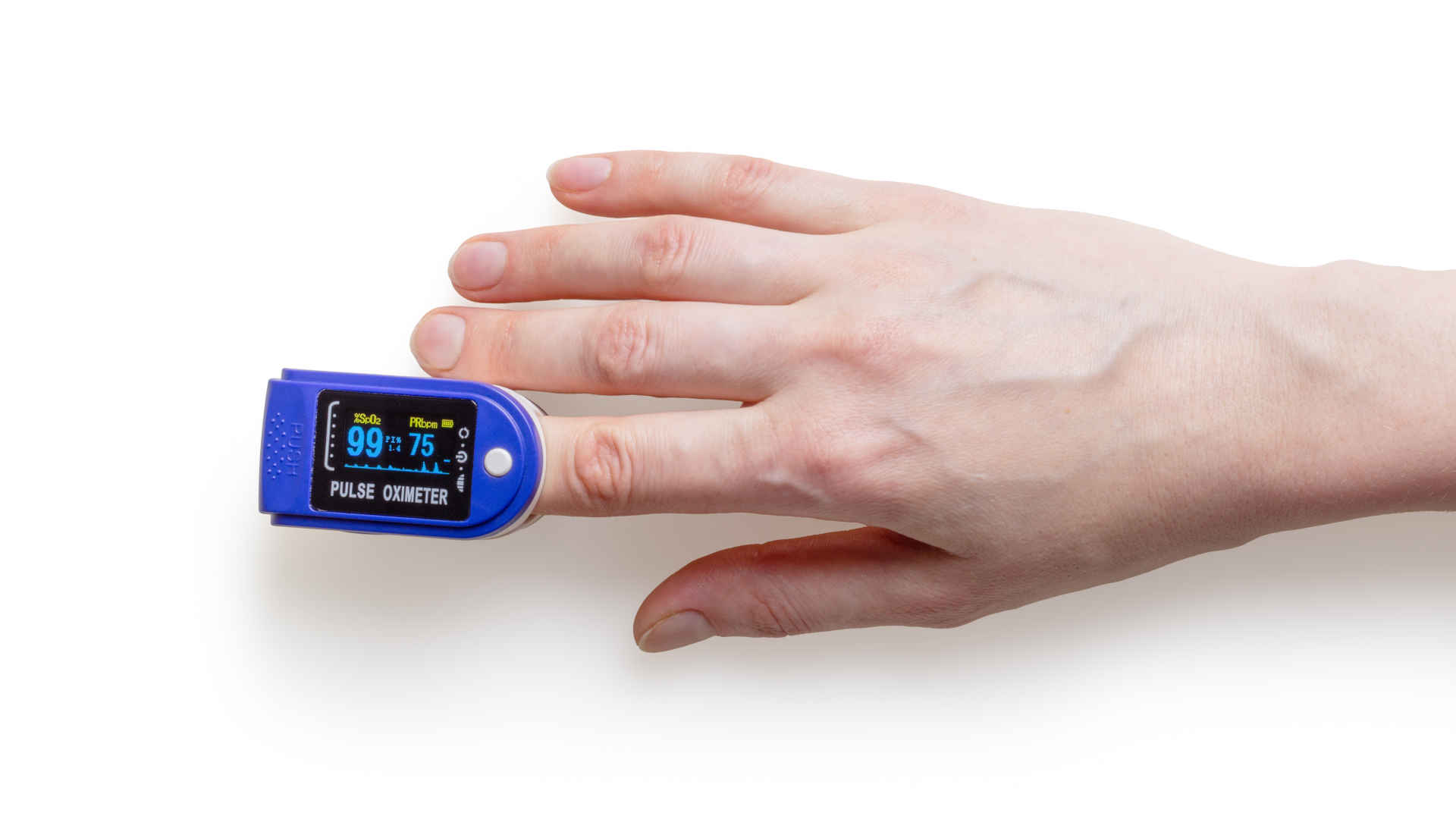
5 Trends Shaping the Future of Advanced Medical Devices
Artificial intelligence, surgical robots, custom prosthetics printed on-site, tiny devices sending information from inside the body to medical teams miles away – we live in a time when so much real innovation is happening in the advanced medical device real world that sci-fi may become obsolete! Players in the healthcare landscape, such as advanced medica
Reducing AI Risk to Secure Regulatory Approval
For most of 2023, the consumer tech space has been flooded with news about AI. As the chart below shows, the advanced medical device industry has been flirting with AI for several years. The implications for advanced medical device development are substantial, especially as regulatory bodies grapple with issues around data privacy and bias. Whether […]
Riding the Innovation Wave: 5 Top Medical Device Companies Known for Innovation
Like the Apollo moon shot in the 1960s to today’s Mars Rover explorations showcase exponential leaps in technology, the medical device field has also evolved at a mind-boggling pace. As new technologies like AI and cloud drive innovation, where should advanced medical device leaders look for direction and inspiration while channeling the vision into the [&hel
Designing for Compliance: QMS & Validation Testing for SaMD vs. Physical Devices
“God is in the details” is an old German proverb that Nietzsche (not surprisingly) paraphrased to the more common saying “the Devil is in the details.” While Nietzsche knew nothing about medical device regulatory compliance and testing, anyone experiencing the journey to FDA approval would agree that the process often feels more devilish than divine. [&
AI and the FDA – What New Guidance Means for AI Innovation in Healthcare
When ChatGPT exploded on the scene in late November 2022, Artificial Intelligence (AI) became the mainstream hot topic of discussion. And for good reason; AI’s potential influence on human society is almost infinite and accelerating rapidly. The healthcare industry is lagging slightly in AI adoption compared to other industries. Even so, connected medical
Choosing the Right API for Your Medical Device
Cloud-based solutions for data storage is not a recent innovation. A relatively new concept in medical devices is storing data in the cloud. Given the mission-critical task of protecting and managing that data, some medical device developers may consider “build your own” solutions as the best approach. This post gives an overview of what to […]
Update on Clinical Decision Support Systems
Technology is revolutionizing healthcare, yet the road to revolution can be bumpy at times. New technologies are blurring the lines between medical devices as gadgets and Software as Medical Device (SaMD). What’s more, healthcare providers face cognitive overload from device complexity and an increase in patient data. At the same time, the demand for indi
Fast Healthcare Interoperability Resources (FHIR), What It Is and Why You Should Care
In the 1960s record keeping began to shift from handwritten notes on paper charts to transcribed information into electronic healthcare records or EHR. At its inception, EHR was so expensive only the government and large healthcare organizations could afford the transition. The arrival of the internet created a demand for sharing EHR, as well as […]
Trends driving demand for advanced medical devices for outpatient care.
The healthcare industry is undergoing a significant transformation, driven by various factors such as the need for cost savings and a shortage of personnel. As a result, advanced medical devices are increasingly vital in providing efficient and effective care in outpatient settings. Let’s take a closest look at the key trends driving the demand for [&hell
How to Proactively Manage Risk for Advanced Medical Device Manufacturers
Advanced medical devices are revolutionizing patient outcomes by providing innovative solutions to challenging conditions. Even so, regulatory agencies and advanced medical device companies are fully aware that devices can pose significant patient risks in the product life cycle from faulty design, development, or manufacturing. Proactively managing risk is cru